Documentation:CHBE Exam Wiki/Midterm Exam 2 2016W/MT2 Question 1
| CHBE 241 Exam resources wiki | |
|---|---|

| |
| Chemical and Biological Engineering | |
|
Welcome to the CHBE Exam Resources Wiki!
This wiki is intended to host past exams | |
| Past Exams | |
| Final Exam 2016W | |
| Midterm Exam 1 2016W | |
| Midterm Exam 2 2016W | |
| Problem Sets | |
| Module 1 - Process Basics | |
| Module 2 - Reactors | |
| Module 3 - Separations 1 | |
| Module 4 - Separations 2 | |
| Module 5 - Non-reactive Energy Balances | |
| Module 6 - Reactive Energy Balances | |
Ethylene oxide is a widely used feedstock in chemical synthesis produced at rates of over 20 million tonnes per year. It can be produced by the oxidation of ethylene under a silver catalyst. This reaction also competes with the complete combustion of ethylene. A block diagram for an ethylene oxide plant is shown below, with the species indicated in each stream along with the reaction for the synthesis of ethylene oxide from ethylene.
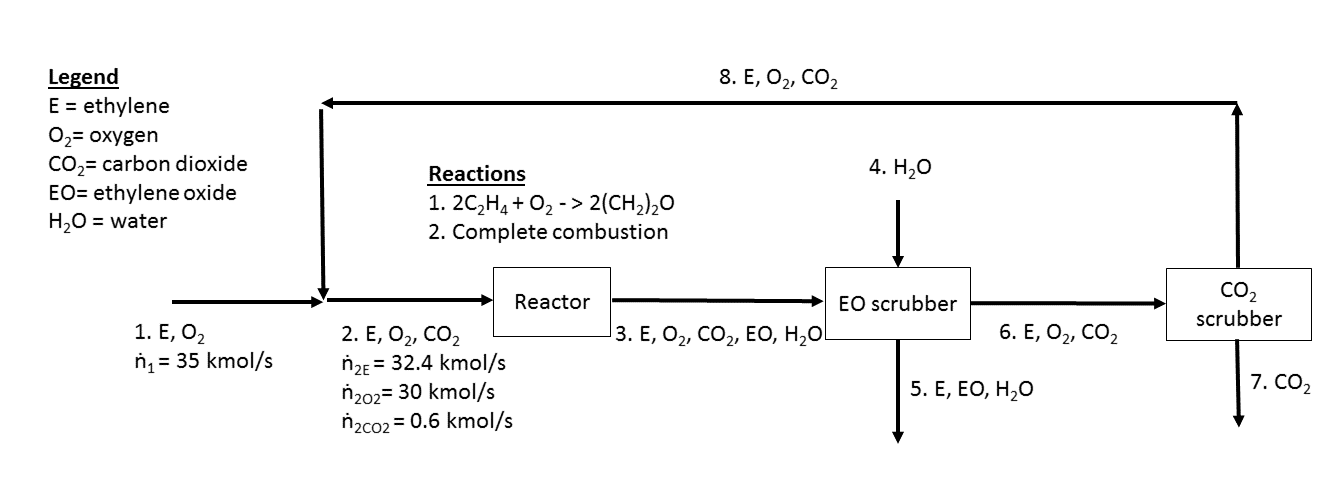
Question 1a [5 points]
If the single pass conversion of ethylene is 40% when considering only the desired reaction for producing ethylene oxide, what is the exiting flow of ethylene oxide from the reactor (in kmol/s)?
Hint |
|---|
|
How does conversion relate to the inlet and outlet flow rates of ethylene? |
Solution |
|---|
|
|
Question 1b [10 points]
Considering both the desired and undesired combustion reaction now, the selectivity of ethylene oxide to CO2 formed is 2.28. What is the exiting flow of CO2 from the reactor (in kmol/s)?
Hint |
|---|
|
How does the selectivity relate the desired and undesired product flows? |
Solution |
|---|
|
The combustion reaction is The undesired product flow = desired product / selectivity
|
Question 1c [20 points]
If the ethylene oxide (EO) scrubber removes 10% of the entering ethylene (E) and the CO2 scrubber removes 90% of the entering CO2, what is the molar recycle ratio (flow of recycle/flow of fresh feed) for this process?
Hint |
|---|
|
Step 1 Determine the extents of reaction for the two reactions to find the flow rate of the components in stream 3 Step 2
Determine the flow rate of the components in stream 8 and subsequently the flow rate of stream 8 |
Solution |
|---|
|
Reaction 1:
Step 1 Based on the reactions above, the extents of reaction are: Determine the flow rate of ethylene and oxygen in stream 3,
Step 2
Determine the flow rate of stream 8
Recycle ratio |
Script error: The function "navbox" does not exist.