Documentation:CHBE Exam Wiki/Module 6 - Reactive Energy Balances
| CHBE 241 Exam resources wiki | |
|---|---|

| |
| Chemical and Biological Engineering | |
|
Welcome to the CHBE Exam Resources Wiki!
This wiki is intended to host past exams | |
| Past Exams | |
| Final Exam 2016W | |
| Midterm Exam 1 2016W | |
| Midterm Exam 2 2016W | |
| Problem Sets | |
| Module 1 - Process Basics | |
| Module 2 - Reactors | |
| Module 3 - Separations 1 | |
| Module 4 - Separations 2 | |
| Module 5 - Non-reactive Energy Balances | |
| Module 6 - Reactive Energy Balances | |
Question 1
Trichloroethylene is produced in a two-step reaction sequence as shown in the steps below.
Reaction 1: C2H4(g) 2Cl2 (g) → C2H2Cl4(l) + H2(g) where,
Reaction 2: C2H2Cl4(l) → C2HCl3(l) + HCl(g)
The standard heat of formation of tricholoroethylene (liquid) is - 276 2 kJ/mol.
Question 1a
Calculate the standard heat of the second reaction.
We first need to determine the heat of formation of tetrachloroethane
Question 1b
Use Hess’s law to calculate the standard heat of the reaction
C2H4(g)+ 2Cl2 (g) → C2HCl3(l) + H2(g) + HCl(g)
Reaction (1) + Reaction (2)
Question 1c
If 450 mol/h of C2HCl3 (l) is produced in the reaction of part (b) and the reactants and products are all at 25 C and 1 atm, how much heat is evolved or absorbed in the process?
Assume that
Question 2
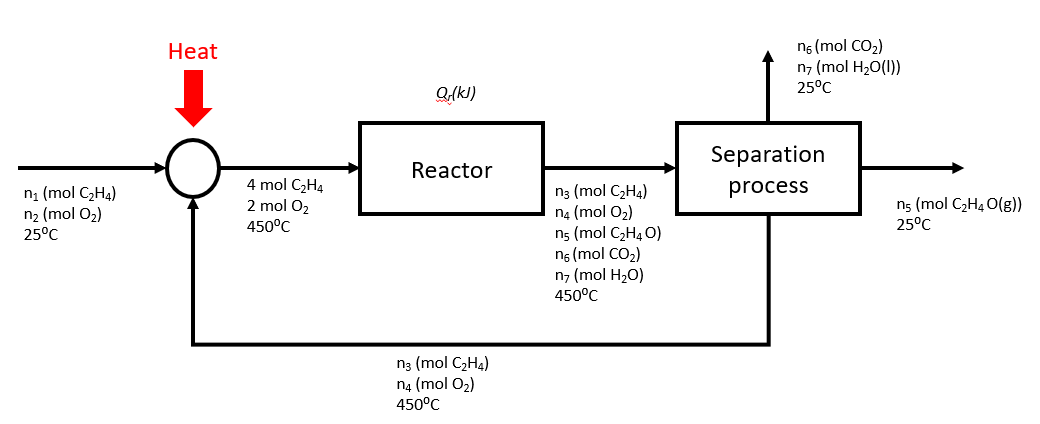
Ethylene oxide is produced by the oxidation of ethylene under the following reaction:
C2H4(g) + 1/2 O2 (g) → C2H4O(g)
Another competing reaction results in oxidation of ethylene to CO.
In a plant, the feed to the reactor contains 2 mol C2H4/ mol O2 while the yield and the conversion in the reactor are 0.8 mol C2H4O produced/ mol C2H4 consumed and 40% respectively. The outlet stream from the reactor then separates into the following streams:
- C2H4 and O2 are recycled back to the reactor
- C2H4O is sold
- CO2 and H2O are disposed
Also, the inlet and outlet streams from the reactor are kept at 450°C, while the feed stream and the streams leaving the separator are at 25°C.
Question 2a
Using a basis of 4 moles of ethylene entering the reactor, draw a process flow diagram for the plant.
* Note that the recycle stream above should be at 25°C according to the question statement.
Question 2b
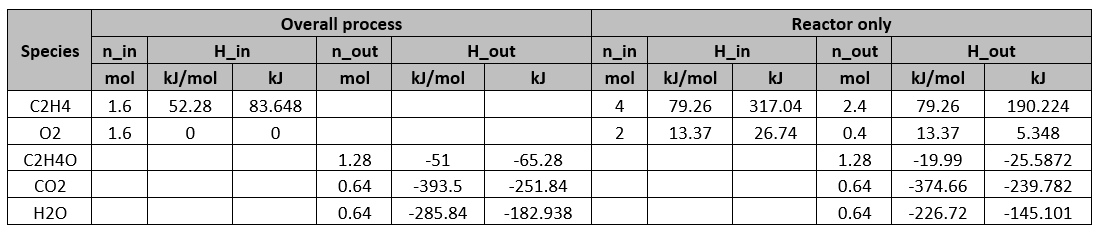
Determine the flow rates (molar) and compositions of all the streams.
With 40% conversion, 1.6 mol of C2H4 is consumed. Therefore,
With 80% yield,
C balance on reactor:
Since the molar ratio of water to CO2 produced is 1:1,
O balance on the reactor:
Overall C balance:
Overall O balance:
Compositions:
Feed stream: 50% C2H4, 50% O2
Recycle stream: 85.7% C2H4,14.2% O2
Reactor inlet: 66.7% C2H4, 33.3% O2
Reactor outlet: 44.8% C2H4, 7.5% O2, 23.9% C2H4O, 11.9% CO2, 11.9% H2O
Question 2c
Determine the heat required for the whole process and the reactor alone respectively. Use the following information for C2H4O,
where T is in kelvin
Question 2d
Determine the flow rate (kg/h) and the energy consumption in kW for a production for 5000kg C2H4O/day.
Mass of C2H4O produced with initial basis,
The feed mass at the initial basis,
The fresh feed rate for the production basis of 5000kg/day,
By performing energy balance on the process:
Question 3
In a continuous reactor at 25°C, dilute sulphuric acid was used to absorb ammonia which results in the formation of ammonium sulfate,
2 NH3(g) + H2SO4(aq) → (NH3)2SO4 (aq)
Question 3a
If the product solution is at 25°C while the ammonia and sulfuric acid feeds are at 75°C and 25°C respectively, how much heat must be withdrawn from the unit per mol of (NH3)2SO4 produced?
By setting the reference to be at 25°C and the basis to be 1 mol of (NH3)2SO4 produced,
Ammonia(g,75°C)
H2SO4(aq,25°C)
(NH4)2SO4(aq,25°C)
Energy balance,
177 kJ should be removed from the unit per mol of (NH3)2SO4 produced.
Question 3b
If the product stream contains 1 mole% ammonium sulfate, estimate the final temperature of the reactor.
Assume that there is no heat loss by the reactor and the heat capacity of the solution to be that of pure liquid water [4.184 kJ/(kg C)].
Determine the total mass of the solution using a basis of 100 moles.
Since there is not heat lost to the surroundings, the heat transferred from the reactor is the heat absorbed by the solution, resulting in temperature change.
Question 4
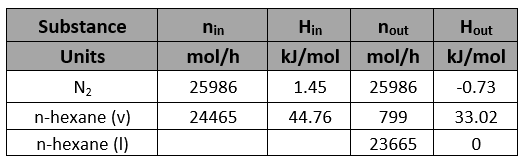
A gas mixture containing n-hexane in nitrogen with a relative saturation of 80% is fed to a condenser where the feed stream is at 75°C and 2 atm (absolute). Two streams leave the condenser where one stream is in the gas phase while the other stream is in liquid phase. The product gas stream leaves the condenser at 0°C and 2 atm (absolute) at a flow rate of 300 m3/h.
Question 4a
Determine the percentage condensation of hexane (moles condensed/mole fed).
The molar flow rate of the gas stream,
Determine the sat. vapor pressure of n-hexane(h) in both the gas streams (inlet and outlet).
Determine the vapor fraction of n-hexane(h) in both the gas streams (inlet and outlet).
Determine the molar flow rates of the feed and liquid streams
N2 balance:
n-hexane balance:
Question 4b
Determine the rate of heat transfer (kW) from the condenser.
Question 5
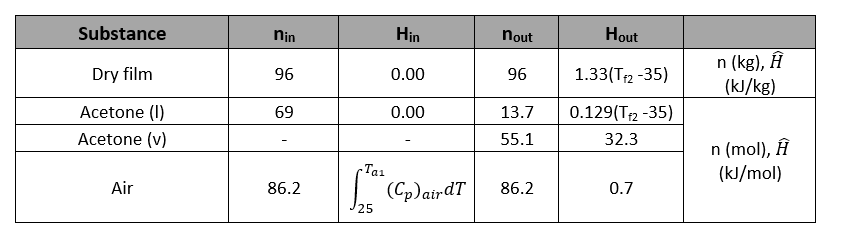
In an adiabatic dryer, a sheet of film which contains 4 wt% liquid acetone enters where 80% of the acetone evaporates into air. The film enters at 35°C (Tf1) and exits at Tf2 while air enters at Ta1 and leaves at 49°C with relative saturation of 50%. The air enters at 1.01 atm and leaves the dryer at 1 atm
Also, use these following assumptions:
- Cp for dry film is 1.33 kJ/(kg °C)
- Cp for liquid acetone is 0.129 kJ/(mol °C)
- The heat of vaporization of acetone is independent of temperature
Question 5a
Calculate the feed ratio [liters dry air (STP)/kg dry film] by assuming a basis of 100kg of film being fed into the dryer.
Determine the sat. vapor pressure of acetone
Determine the molar flow rate of acetone in exit gas,
Determine the vapor fraction of n acetone in exit gas,
Determine the molar flow rates of the feed air
Question 5b
Derive an expression for Ta1 in terms of the film temperature change, (Tf2 - 35)
Question 5c
Calculate the film temperature change if the inlet air temperature is 120°C.
If Ta1 is 120°C,