Documentation:CHBE Exam Wiki/Final Exam 2016W/Question 3
| CHBE 241 Exam resources wiki | |
|---|---|

| |
| Chemical and Biological Engineering | |
|
Welcome to the CHBE Exam Resources Wiki!
This wiki is intended to host past exams | |
| Past Exams | |
| Final Exam 2016W | |
| Midterm Exam 1 2016W | |
| Midterm Exam 2 2016W | |
| Problem Sets | |
| Module 1 - Process Basics | |
| Module 2 - Reactors | |
| Module 3 - Separations 1 | |
| Module 4 - Separations 2 | |
| Module 5 - Non-reactive Energy Balances | |
| Module 6 - Reactive Energy Balances | |
A furnace is used in a plant to heat a stream of 1 tonne per hour of pure H2O. The H2O enters as a mixture of water (liquid) and steam (vapour) at 5 bar to produce superheated steam at 300°C and 5 bar. The heat to do this is provided by the complete combustion of 1 kmol/h propane (C3H8) with 100% excess air at 1 atm. The air consists of 79 mol% nitrogen (N2) and 21 mol% oxygen (O2) and enters the furnace with the propane at 200°C. The fractional conversion of propane is 1.00 and the combustion products leave the furnace at 400°C. The heat of propane combustion forming gaseous water is -2220 kJ/(mol C3H8) at 400 °C. The steam and combustion streams are not in direct contact, but energy is transferred between the two streams and you can assume the furnace to be adiabatic with negligible energy contributions from shaft work, kinetic energy, or potential energy.
| Compound | Cp[J/mol K] |
|---|---|
| C3H8 | 68 |
| N2 | 29 |
| H2O | 33.5 |
| O2 | 29 |
| CO2 | 36 |
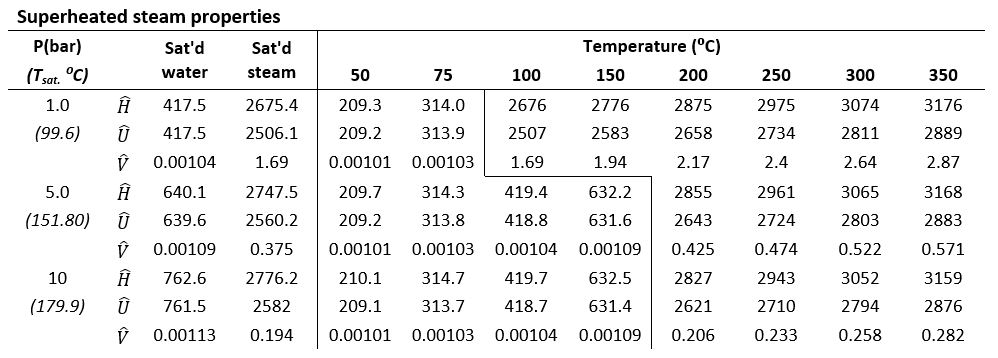
Note : enthalpy and internal energy values below are in kJ/kg and volumes are in m3/kg
Question 3a [5 points]
Write out the equation for the combustion reaction. What is the molar flow of oxygen and nitrogen into the process?
Hints |
|---|
|
Solution |
|---|
|
Combustion reaction Using stoichiometry, the ratio of propane to oxygen is 1:5. Since air is fed with 100% excess to the furnace, the ratio is doubled. Hence, The composition of nitrogen and oxygen in air are 79 mol% and 21 mol% respectively. Hence, |
Question 3b [10 points]
What are the molar fractions of all species in the gas exiting the furnace?
Hints |
|---|
|
Determine the molar flow rates of the each species exiting the furnace by taking into account the extent of reaction (conversion) |
Solution |
|---|
|
Step 1 Reactant species: Propane and oxygen Conversion of propane is 1.00. Hence
Step 2
The molar fractions of the species in the exit stream of furnace are: |
Question 3c [15 points]
What is the amount of energy transferred due to the combustion reaction in kJ/h?
Hints |
|---|
|
Step 1 How does the temperature change in the streams relate to the change in enthalpy? Step 2
How does energy balance come into play in this situation? |
Solution |
|---|
|
Step 1 Take the reference temperature, to be Assuming that the value is independent of temperature and pressure, the molar enthalpy is given by the following formula Determine the molar enthalpy for all the species
Step 2
Determine the energy transfer using energy balance |
Question 3d [10 points]
What are the weight fractions of water and steam in the entering pure H2O?
Hints |
|---|
|
Step 1 Look for the appropriate values in the steam table based on the stream conditions Step 2
How can the energy balance involving specific enthapy be applied in this situation? |
Solution |
|---|
|
Step 1 From the steam table, the following values are obtained Let the fraction of liquid water be Step 2
Using energy balance Rearranging the expression and solving for |
Script error: The function "navbox" does not exist.



























