Documentation:CHBE Exam Wiki/Final Exam 2016W/Question 1
| CHBE 241 Exam resources wiki | |
|---|---|

| |
| Chemical and Biological Engineering | |
|
Welcome to the CHBE Exam Resources Wiki!
This wiki is intended to host past exams | |
| Past Exams | |
| Final Exam 2016W | |
| Midterm Exam 1 2016W | |
| Midterm Exam 2 2016W | |
| Problem Sets | |
| Module 1 - Process Basics | |
| Module 2 - Reactors | |
| Module 3 - Separations 1 | |
| Module 4 - Separations 2 | |
| Module 5 - Non-reactive Energy Balances | |
| Module 6 - Reactive Energy Balances | |
The water gas shift reaction is commonly used for producing hydrogen and follows the reaction given below:
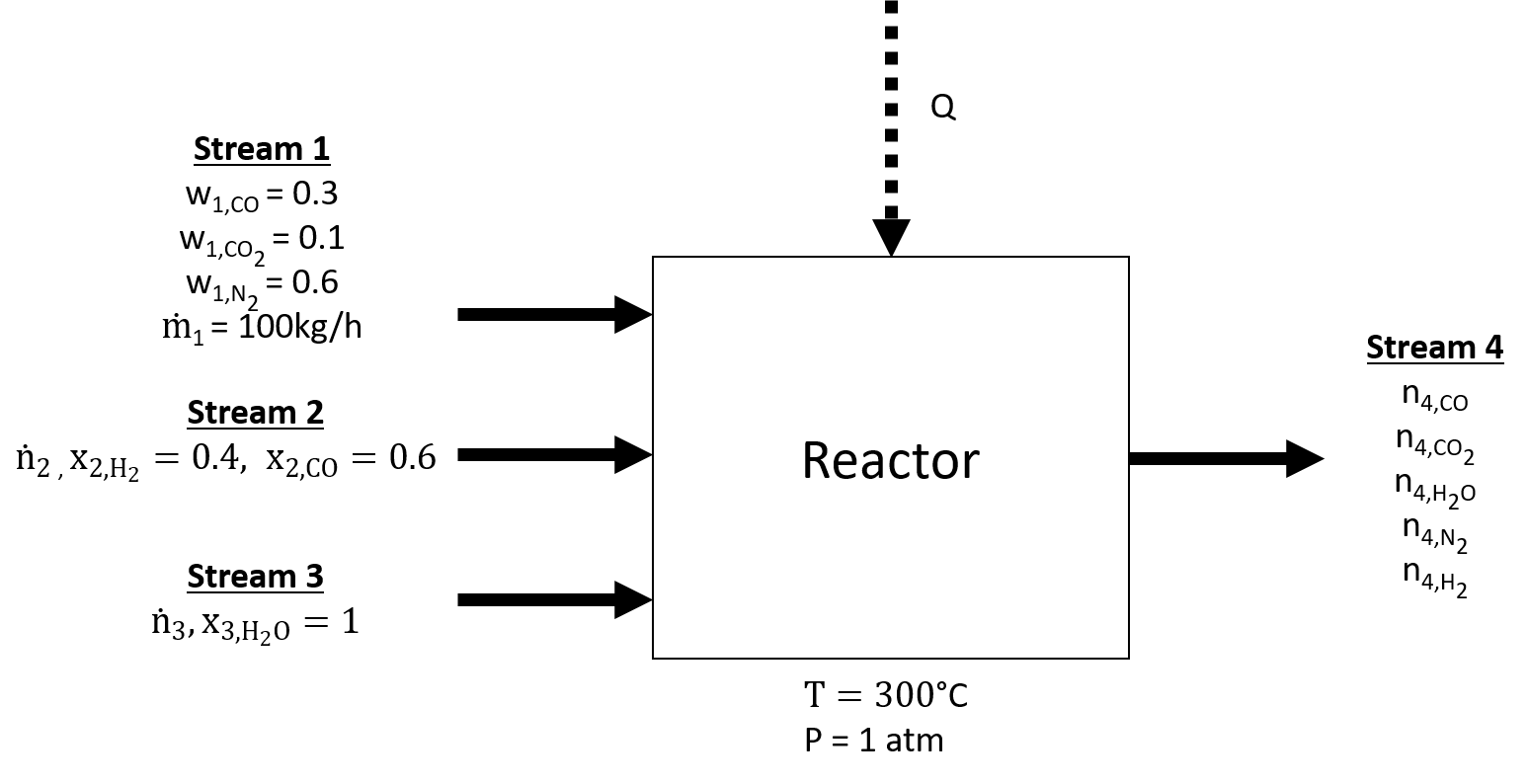
Say we have a water gas shift reactor with 3 entering streams. The first stream (#1) contains 10 weight% CO2, 30 weight% CO and the remainder as nitrogen (N2) and enters at 100 kg/h. The second stream (#2) contains 40 mol% hydrogen and the remainder as CO. The third entering stream (#3) contains only water. Only one product stream exits and it contains a mixture of all the entering species. The reactor achieves an 80% conversion of the entering CO. The reactants and products enter and leave at 300 °C and 1 atm and the reactor exchanges energy by giving off or taking in heat to achieve this. The exiting stream is not under equilibrium.
Physical Data
MW CO2 : 44 g/mol
MW CO : 28 g/mol
MW N2 : 28 g/mol
Question 1a [5 points]
Draw a flowchart for the process labeling all streams and components.
Hint |
|---|
|
Solution |
|---|
Question 1b [5 points]
Indicate the mole fractions of all components and the total molar flow in kmol/h for stream #1.
Hint |
|---|
|
Solution |
|---|
|
|
Question 1c [5 points]
Do a degrees of freedom analysis on the reactor. Is the problem over-specified, under-specified or adequately specified?
Hint |
|---|
|
How many components are in each stream and how many flows are known? Don't forget to include an energy balance. |
Solution | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Since the degrees of freedom is positive, it is underspecified. |
Question 1d [5 points]
The pressure gauge for the reactor was found to not be accurate and instead a manometer was installed with one end attached to the reactor and then other end open to the atmosphere. A liquid with a specific gravity of 5.27 (relative to water at 4°C) was used in the manometer. The atmospheric pressure was found to be 100 kPa. The fluid was higher on the side open to the atmosphere by 50 cm. What is the absolute pressure inside the reactor in kPa?
Hint |
|---|
|
Solution |
|---|
|
|
Script error: The function "navbox" does not exist.