Documentation:CHBE Exam Wiki/Module 6 - Reactive energy Balances
| CHBE 241 Exam resources wiki | |
|---|---|

| |
| Chemical and Biological Engineering | |
|
Welcome to the CHBE Exam Resources Wiki!
This wiki is intended to host past exams | |
| Past Exams | |
| Final Exam 2016W | |
| Midterm Exam 1 2016W | |
| Midterm Exam 2 2016W | |
| Problem Sets | |
| Module 1 - Process Basics | |
| Module 2 - Reactors | |
| Module 3 - Separations 1 | |
| Module 4 - Separations 2 | |
| Module 5 - Non-reactive Energy Balances | |
| Module 6 - Reactive Energy Balances | |
Question 1
Trichloroethylene is produced in a two-step reaction sequence as shown in the steps below.
Reaction 1: C2H4(g) 2Cl2 (g) → C2H2Cl4(l) + H2(g) where,
Reaction 2: C2H2Cl4(l) → C2HCl3(l) + HCl(g)
The standard heat of formation of tricholoroethylene (liquid) is - 276 2 kJ/mol.
Question 1a
Calculate the standard heat of the second reaction.
We first need to determine the heat of formation of tetrachloroethane
Question 1b
Use Hess’s law to calculate the standard heat of the reaction
C2H4(g)+ 2Cl2 (g) → C2HCl3(l) + H2(g) + HCl(g)
Reaction (1) + Reaction (2)
Question 1c
If 450 mol/h of C2HCl3 (l) is produced in the reaction of part (b) and the reactants and products are all at 25 C and 1 atm, how much heat is evolved or absorbed in the process?
Assume that
Question 2
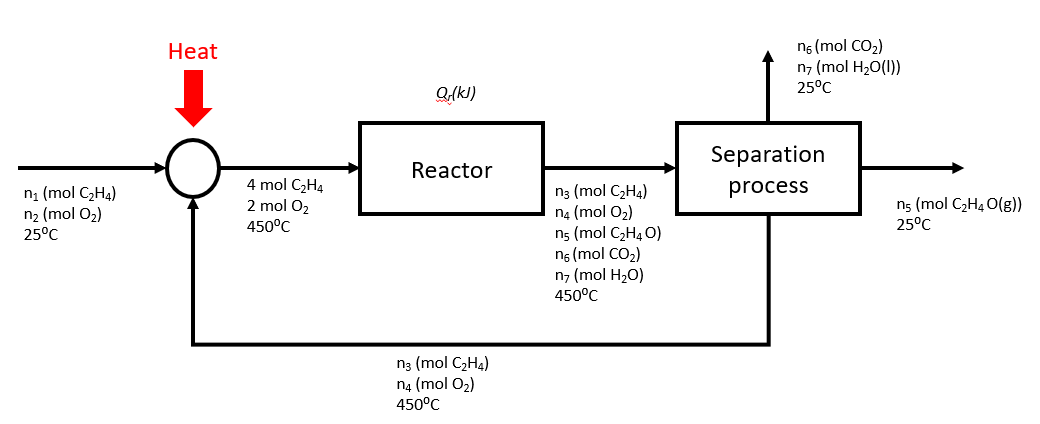
Ethylene oxide is produced by the oxidation of ethylene under the following reaction:
C2H4(g) + 1/2 O2 (g) → C2H4O(g)
Another competing reaction results in oxidation of ethylene to CO.
In a plant, the feed to the reactor contains 2 mol C2H4/ mol O2 while the yield and the conversion in the reactor are 0.8 mol C2H4O produced/ mol C2H4 consumed and 40% respectively. The outlet stream from the reactor then separates into the following streams:
- C2H4 and O2 are recycled back to the reactor
- C2H4O is sold
- CO2 and H2O are disposed
Also, the inlet and outlet streams from the reactor are kept at 450°C, while the feed stream and the streams leaving the separator are at 25°C.
Question 2a
Using a basis of 4 moles of ethylene entering the reactor, draw a process flow diagram for the plant.
Question 2b
Determine the flow rates (molar) and compositions of all the streams.
With 40% conversion, 1.6 mol of C2H4 is consumed. Therefore,
With 80% yield,
C balance on reactor:
Since the molar ratio of water to CO2 produced is 1:1,
O balance on the reactor:
Overall C balance:
Overall O balance:
Compositions:
Feed stream: 50% C2H4, 50% O2
Recycle stream: 85.7% C2H4,14.2% O2
Reactor inlet: 66.7% C2H4, 33.3% O2
Reactor outlet: 44.8% C2H4, 7.5% O2, 23.9% C2H4O, 11.9% CO2, 11.9% H2O
Question 2c
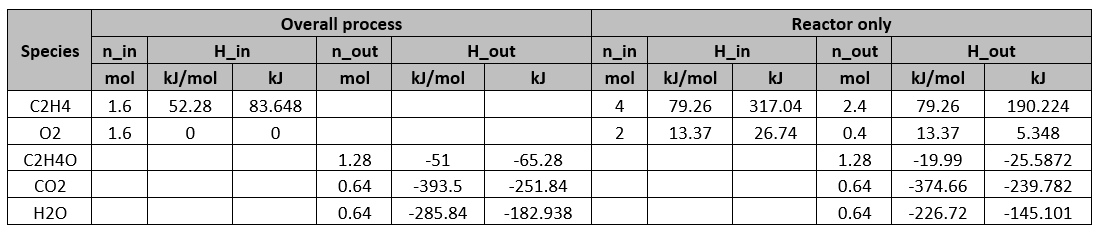
Determine the heat required for the whole process and the reactor alone respectively. Use the following information for C2H4O,
where T is in kelvin
Question 2d
Determine the flow rate (kg/h) and the energy consumption in kW for a production for 5000kg C2H4O/day.
Mass of C2H4O produced with initial basis,
The feed mass at the initial basis,
The fresh feed rate for the production basis of 5000kg/day,
By performing energy balance on the process: