Course:MEDG550/Student Activities/Zellweger Spectrum Disorder
Zellweger spectrum disorder (ZSD) is a type of peroxisome biogenesis disorder (PBD), meaning there is an absence of normal peroxisomes in the cells of the body [1]. Because peroxisomes play a role in many areas of the human body, disorders of this kind can be progressive and affect multiple systems. There is a broad spectrum of severity for Zellweger spectrum disorder, and it can be subdivided into three groups: Zellweger syndrome (ZS), infantile refsum disease (IRD), and neonatal adrenoleukodystrophy (NALD)[2]. The prevalence of these congenital disorders is estimated to be around 1/50,000[3].
Medical features
Zellweger syndrome (ZS)
Also known as cerebrohepatorenal syndrome, this is the most severe form of ZSD[4]. Symptoms of this condition are usually apparent within the first few hours or days of life and include weak muscle tone (hyptonia), feeding problems, hearing loss, vision loss, and seizures. Individuals affected by this disorder also have characteristic facial features, with a flattened face, broad nasal bridge, and high forehead. Children develop severe liver, kidney and heart problems, and may have skeletal abnormalities. Children with Zellweger syndrome do not typically survive beyond the first year of life[2].
Neonatal adrenoleukodystrophy (NALD) and Infantile refsum disease (IRD)
These disorders are similar to ZS but are less severe and tend to progress over time[4]. Symptoms present in late infancy or early childhood and are more variable. Common features are hypotonia, failure to thrive, seizures, vision problems, hearing loss, liver dysfunction, developmental delay, and variable intellectual disability. People with NALD typically survive into childhood, and those with IRD may reach adulthood and develop some degree of communication, cognitive and motor skills[2].
Causes
Peroxisomes are small organelles (internal cellular structures that perform tasks for the cell) that play a role in a variety of cell functions[5]. The two main functions of the peroxisome is to break down fatty acids (for metabolic energy), and to create hydrogen peroxide from oxygen and later converting that into water[5]. Because peroxisomes are involved in cells all over the body, peroxisome dysfunction can lead to a multiple organ system disorder[4].
Normal peroxisome function depends on 16 proteins, known as peroxins. Peroxins are encoded by PEX genes. A gene is a piece of genetic information, or DNA, that encodes for specific proteins. If a change is found in a gene encoding one of 13 of these peroxins, this can result in ZSD[6].
| Gene Symbol | % of ZSD Attributed to Variants in this Gene[7] |
|---|---|
| PEX1 | 60.5 |
| PEX6 | 14.5 |
| PEX12 | 7.6 |
| PEX26 | 4.2 |
| PEX10 | 3.4 |
| PEX2 | 3.1 |
| PEX5 | 2.0 |
| PEX13 | 1.5 |
| PEX16 | 1.1 |
| PEX3 | 0.7 |
| PEX19 | 0.6 |
| PEX14 | 0.5 |
| PEX11β | 0.1 |
Diagnosis and testing
Biochemical testing
This test measures levels of certain analytes in blood and/or urine and can be used to definitively diagnose an individual with ZSD[2]. These include measurements of levels of very-long-chain fatty acids (VLFCA), phytanic acid, pristanic acid, plasmalogens, pipecolic acids, and bile acids.
Biochemical abnormalities in blood and/or urine can be confirmed in cultured fibroblasts.
Molecular genetic testing
DNA sequencing can be used to identify the genetic cause in individuals. Because of the large number of genes that may be affected, The PEX Gene Screen was developed [8]. This systematically looks at the DNA sequences, starting from the most commonly affected genes, and progressing until the causative change is identified.
Management
Currently, treatment involves management of symptoms. This requires surveillance of feeding, vision, liver function, adrenal function, as well as neurological, orthopedic, and developmental assessment. A multidisciplinary approach to address these issues may include a variety of health care professionals, and should include a geneticist, neurologist, and nutritionist from early on[4].
It is important for individuals affected with ZSD to have adequate calorie intake as many children have some degree of malabsorption. Gastronomy tubes are usually used to provide elemental formulas and allow for home management of dietary needs. Supplementation of vitamin K and other fat soluble vitamins is also recommeneded. Children who experience hearing loss are treated with hearing aids dependign on their level of impairment. Vision problems can occur such as early onset cataracts, these can be removed in early infancy to preserve vision. Children with ZSD often need glasses to correct refractive errors. Primary bile acid therapy may be used to reduce the accumulation of cholestanoic acids[2].
1/3 individuals affected with ZSD experience seizures which are treated with standard epileptic drugs (AEDs) and no type of AED is contraindicated in these cases[2].
Although the clinical impact of dietary modifications are mostly anecdotal, dietary interventions have been recommended that restrict metabolites that accumalate (such as phytanic acid) and replace those that are deficient (such as plasmalogen precursors, bile acid, and DHA).
Longer surviving individuals should be monitored for hyperoxaluria (increased oxalate in the urine), to prevent renal failure due to stone formation[2].
Individuals should have their hearing and vision evaluated annually. Liver function should be monitored carefully. Neuroimaging can be used to monitor progressgive leukodystrophy. Although no therapy exists, identifying white matter changes can be used to explain cognitive and motor abilities[2].
Genetic counselling
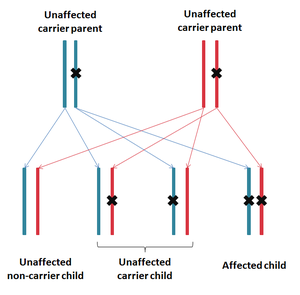
Mode of inheritance
ZSD is inherited in an autosomal recessive fashion, meaning that both copies of the gene carry a mutation in an affected individual. Parents of an affected individual are known as carriers, as they each carry one copy of the mutated gene, but do not show signs or symptoms.
Risk to family members
Carrier testing
If the disease causing mutation is known in the family, other family members that are at risk of being carriers can also be identified.
Recurrence risk
For a couple that both carry a mutation, for each pregnancy there is a:
- 25% chance of being affected (passing on both mutations)
- 50% chance of being unaffected carriers (one mutant copy and one normal copy of the gene)
- 25% chance of being unaffected and not a carrier (two normal copies of the gene)
Family planning
Prenatal diagnosis
Because of the risk for having an affected child, parents that are both carriers are eligible for prenatal diagnosis using biochemical testing or molecular genetic testing.
PGT-M
Preimplantation genetic testing for monogenic/single gene defects (PGT-M) is another option that exists for couples in which their carrier status is known. This is a process that uses in vitro fertilization to produce embryos from a mother's egg and father's sperm. These embryos are then tested for known mutations in the gene and only unaffected embryos will be implanted.
Egg or sperm donation
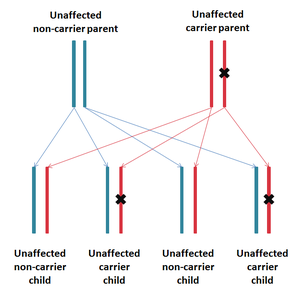
Alternatively, couples may be interested in receiving a donor egg or sperm. Since it is an autosomal recessive disease, even one carrier parent with one non-carrier parent have no risk of having an affected offspring.
Resources
References
- ↑ Gould SJ, Raymond GV, Valle D. The peroxisome biogenesis disorders. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. 8 ed. New York, NY: McGraw-Hill; 2001:3181-218
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Steinberg SJ, Raymond GV, Braverman NE, et al. Peroxisome Biogenesis Disorders, Zellweger Syndrome Spectrum. 2003 Dec 12 [Updated 2012 May 10]. In: Pagon RA, Adam MP, Bird TD, et al., editors. GeneReviews™ [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2014. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1448/
- ↑ Steinberg, S.J., Dodt, G., Raymond, G.V., Braverman, N.E., Moser, A.B., and Moser, H.W. (2006). Peroxisome biogenesis disorders. Biochimica et biophysica acta 1763, 1733-1748.http://www.ncbi.nlm.nih.gov/pubmed/17055079
- ↑ 4.0 4.1 4.2 4.3 Braverman, N.E., D'Agostino, M.D., and Maclean, G.E. (2013). Peroxisome biogenesis disorders: Biological, clinical and pathophysiological perspectives. Developmental disabilities research reviews 17, 187-196. http://www.ncbi.nlm.nih.gov/pubmed/23798008
- ↑ 5.0 5.1 Cooper, GM (2000). The Cell: A Molecular Approach. 2nd edition. Sinauer Associates. pp. Peroxisomes.
- ↑ Braverman, N.E., D'Agostino, M.D., and Maclean, G.E. (2013). Peroxisome biogenesis disorders: Biological, clinical and pathophysiological perspectives. Developmental disabilities research reviews 17, 187-196. http://www.ncbi.nlm.nih.gov/pubmed/23798008
- ↑ Ebberink, M.S., Mooijer, P.A., Gootjes, J., Koster, J., Wanders, R.J., Waterham, H.R. (January 2011). "Genetic classification and mutational spectrum of more than 600 patients with a Zellweger syndrome spectrum disorder". Human mutation. 32(1): 59–69. doi:10.1002/humu.21388.CS1 maint: multiple names: authors list (link)
- ↑ Steinberg, S., Chen, L., Wei, L., Moser, A., Moser, H., Cutting, G., and Braverman, N. (2004). The PEX Gene Screen: molecular diagnosis of peroxisome biogenesis disorders in the Zellweger syndrome spectrum. Molecular genetics and metabolism 83, 252-263.http://www.ncbi.nlm.nih.gov/pubmed/15542397