Course:MEDG550/Student Activities/Wilson Disease
Wilson Disease is a rare genetic condition in which the body is unable to properly transport copper, resulting in a toxic build-up in certain tissues and organs. Copper plays an important role in the body, but excessive amounts can be damaging to the liver, brain and eyes, and can eventually lead to liver disease, neurological symptoms, and psychiatric issues.[1][2] Wilson Disease has a varying age of onset (the age at which symptoms first appear), with individuals diagnosed anywhere between the ages of 3 and 50 years old.[1] It is estimated that Wilson Disease affects approximately 1 in 30,000 people, though some studies estimate it could be as frequent as 1 in 5000 people, depending on the population.[3][4] It can affect both males and females equally.
History
The condition was first described by Kinnear Wilson in 1912 as a familial neurological and chronic liver disease.[2] It was not until the use of DNA linkage studies in 1985, over 70 years later, that the gene involved in Wilson Disease was finally localized to chromosome 13. Following this, researchers in Toronto, Canada were able to use positional cloning techniques to further isolate the specific gene, which was called ATP7B.[4]
Clinical Features
Typically, affected individuals present with a spectrum of liver disease and neuropsychiatric disturbances. They may have all or only some of the symptoms of Wilson Disease, which makes diagnosing the disease difficult.
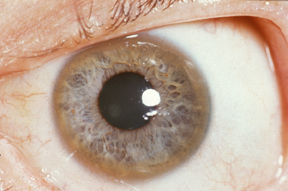
Eye Involvement
Kayser-Fleischer Ring
Although not specific to just Wilson disease, the rusty-brown ring around the edge of the iris, known as the Kayser-Fleischer ring, is considered to be a clinical hallmark feature of this condition.[1][5] These rings result from copper deposits in the cornea and are found in 95% of individuals with neurological or psychiatric symptoms, and approximately 50% of individuals with liver disease.[1][6] A second, much rarer sign of Wilson Disease found in the eyes is the sunflower cataract, which is a greenish-coloured opacity in the centre of the eye that resembles the shape of a sunflower.[7]
Liver Involvement
Generally, any type of liver disease can be present in affected individuals and symptoms can be highly variable, ranging from minor biochemical abnormalities to acute liver failure with associated complications.[2] Other possible presentations include autoimmune-type hepatitis, acute hepatitis, portal hypertension, hepatosplenomegaly fatty liver and cirrhosis.[1][3]
Symptoms include:
- Weakness
- Fatigue
- Recurrent jaundice, a condition that causes yellowing of skin and eyes
- Abdominal pain or swelling
- Rashes
- Nausea
- Loss of appetite
Brain Involvement
Physical Symptoms Include: [3][5]
- Tremors
- Muscle stiffness and rigidity
- Poor physical coordination
- Loss of fine motor control
- Facial motor impairment
Neuropsychiatric Symptoms Include: [3][5][1]
- Changes in personality
- Neurotic behaviours
- Depression
- Anxiety
- Mood swings
- Migraines
- Insomnia
- Psychosis
Other clinical presentations
Other signs and symptoms may include renal abnormalities, arthritis, premature osteoporosis, pancreatitis and cardiomyopathy.[1][2]
Causes
Wilson Disease is caused by a mutation in the ATP7B gene (OMIM# [3]). This gene provides instruction for copper-transporting ATPase found in liver cells and is important in eliminating copper from the liver.[3][8] Nonfunctioning and/or lack of ATP7B protein results in an accumulation of copper within the body. At high levels copper can become toxic and cause damage to tissues and organs.[1][2] To date, there have been than 500 mutations described in ATP7B gene and 380 of these mutations have confirmed to be disease-causing.[3]
Diagnosis
A clinical diagnosis of Wilson Disease is based on a combination of biochemical tests and physical observations and assessment. Confirmation of the clinical diagnosis through genetic testing is also helpful. The presence of Kayser-Fleischer rings and low levels of a copper-carrying protein called ceruloplasmin is enough to establish a clinical diagnosis, though these findings are not required in order to have the diagnosis.
Biochemical Test
Individuals suspected of Wilson Disease will initially undergo various biochemical tests to measure the copper content in their body, as well as the level of copper excretion.[1][2][9] Diagnosing Wilson Disease in newborns and infants can be difficult, since they typically have low levels of the copper-carrying protein ceruloplasmin to begin with.[1] Biochemical testing looks at the following things:
- Serum ceruloplasmin
- 24-hour urinary copper
- Serum free copper
- Hepatic copper
Neurological Assessment
Individuals suspected of having Wilson Disease can also under go an MRI (magnetic resonance imaging) in order to detect any structural abnormalities commonly found with the condition.[9] A physical exam is also performed to assess the presence of a tremor, ataxia, and/or dystonia. [9]
Slit-Lamp Examination
In order to properly identify the Kayser-Fleischer rings that are commonly seen in Wilson Disease, a doctor will use a slit-lamp, which is basically a microscope with a bright light, that allows for closer examination of the eyes.
Genetic Test
When a clinical diagnosis has been given, or if an individual is suspected to have Wilson Disease but it is not certain, molecular testing of the ATP7B gene can be done to provide clarity on the diagnosis, as well as facilitate screening for other at risk family members.[3] It is the only way to confirm Wilson Disease in the neonatal period. [1]
- Prenatal diagnosis is available in pregnancies where both parents are known carriers.
- Carrier testing is available to at-risk family members when a known ATP7B mutation has been identified in a family member.
- At risk family members may be eligible for biochemical assessment, liver function tests, slit lamp examinations and genetic testing if familial mutation is known.
Management and Treatment
Proper management and treatment of Wilson disease is crucial, as the accumulation of copper leads to irreparable damage of tissues and organs, and can be fatal without intervention. This makes early diagnosis and treatment extremely important in preventing tissue damage. Affected individuals are advised to avoid certain foods that are high in copper, such as liver, brain, chocolate, mushrooms, shellfish and nuts.[3] Treatment involves the use of copper bonding (chelating) agents such as penicillamine or trientine to bind with and help remove excess copper.[1][2] This treatment must be continued throughout the affected individual's entire life. Other treatment options include zinc, which can absorb copper from gastrointestinal tract and antioxidants. As treatment is an ongoing process, it is recommended that affected individuals have biannual biochemistry testing, blood work, and physical examinations to ensure they remain healthy.
Transplant
In situations where the affected individual is already suffering from acute liver failure, treatment with copper bonding agents is not an effective strategy. When this happens, the only treatment is a liver transplant.[3]
Clinical Trials
Recent interest surrounding gene therapy has been a topic of discussion. However, gene therapy in mice is currently an ongoing field of research.[10] More information is available at: https://clinicaltrials.gov/ct2/show/NCT02273596?term=WTx101&rank=1.
Genetics

Autosomal Recessive Inheritance Pattern
Wilson Disease is a genetic condition caused by changes in the ATP7B gene. Genes are what make up our genetic information (also known as DNA) and can be thought of as a set of instructions that are used to make proteins and perform all the tasks that the body needs in order to function. Changes or variations in the genes can sometimes result in changes to the proteins, which may not function normally. Humans typically inherit two copies of each gene, with one copy coming from the mother and one from the father.
Wilson Disease is inherited in an autosomal recessive pattern. In order to be affected with Wilson Disease, an individual needs to receive two non-working copies of the ATP7B gene; one copy from their mother and one copy from their father. Individuals with one normal copy and one non-working copy of ATP7B are unaffected and are known as carriers.
Carriers have a 50% chance of passing on the normal copy of the ATP7B gene, and 50% chance of passing on the non-working copy to each of their children. When both parents are carriers, there are three potential outcomes for their children:
- 25% chance of an unaffected, non-carrier child as both parents pass on their normal copy of ATP7B
- 50% chance of an unaffected, carrier child as one parent passes on the normal copy and the other passes on the non-working copy of ATP7B
- 25% chance of a child affected with Wilson Disease as both parents pass on their non-working copy of ATP7B
A small proportion of affected individuals will have only one parent identified as a carrier. In this case, the second mutation may be a de novo mutation, meaning it occurred spontaneously in the sperm or egg or shortly after fertilization. In this case, the recurrence of Wilson Disease in future pregnancies would be less than 1%.
Carriers
Carriers are not affected with Wilson Disease, however some carriers may have slightly abnormal levels of copper in the urine or copper-carrying protein in the blood. These altered copper levels can also be seen in the unaffected population. Therefore, molecular testing of the ATP7B gene is the only way to definitively determine carrier status.[1] Carriers are also at risk of passing on the genetic condition and are advised to attend a genetic counselling appointment to understand more about their risk and family planning.
Genetic Counselling
In order to perform genetic testing for Wilson Disease, or to help confirm the diagnosis, affected individuals and their families are often referred to a Genetic Counsellor. These are health care professionals who are specially trained to help people understand the implications of their genetic diagnosis, and adapt to life with a genetic disorder. They can help interpret medical information, discuss recurrence risks, review genetic testing options, and provide support to people as they navigate difficult decisions. Genetic counselling appointments are generally made through health care professional referrals.
An appointment with a Genetic Counsellor may include some of the following:
- Get to Know You - Check in around what you would like to get from the appointment
- Family History/Medical History/Pregnancy History - Collect information about an individual and their family to better understand who may be at risk for Wilson Disease
- Review Disease Information - Provide detailed information about Wilson Disease and the spectrum of symptoms
- Provide Risk Estimates - Determine the chances of a person having Wilson Disease based on personal and family history
- Facilitate Genetic Testing - Help explain genetic testing options, and provide requisitions to facilitate the testing if appropriate
- Support Decision Making - Provide information, resources, and support to facilitate decision making around genetic testing, and family planning
Considerations for Wilson Disease
The carrier frequency of Wilson Disease is estimated to be approximately 1 in 90 people, however, there are over 500 different variants reported in the ATP7B gene. Most of these disease-causing variants are private (they are unique to a specific family), and are uncommon in the general population. Like in many other autosomal recessive conditions, consanguineous couples (couples who are related by blood to each other) are therefore at increased risk of having a child with Wilson Disease if they both carry the same change in ATP7B. Individuals with two copies of the same variant in ATP7B are said to be homozygous, and would have a diagnosis of Wilson Disease, however, it is also possible to have Wilson Disease if an individual has two different changes in the ATP7B gene on different copies. In this case, the individual is said to be a compound heterozygote.
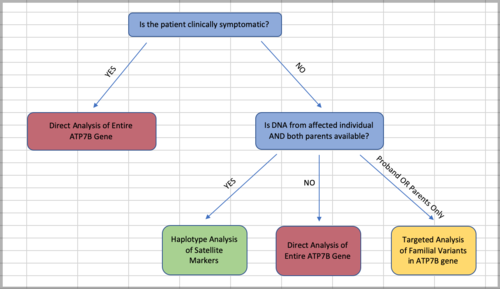
As with other autosomal recessive conditions, Wilson Disease is often the result of both parents being carriers, which means that the siblings of people affected with the disorder are at an increased risk of also having it. For this reason, Genetic Counsellors can help facilitate genetic testing of these at-risk family members. The flow chart depicted in the image above shows how the type of genetic testing can be determined based on availability of DNA from family members, and taking accessibility and cost into account. Typically, an individual affected with Wilson Disease will have direct gene sequencing of the entire ATP7B gene to determine the presence of changes that might cause a non-functional protein. However, the gene is quite large which makes sequencing and analyzing the whole thing expensive and time-consuming. Because of this, if there are known familial variants already, it is preferable to perform the less expensive targeted testing in asymptomatic at-risk relatives to determine whether they carry the same familial variants. It is also possible to perform the less expensive haplotype analysis, without directly sequencing the ATP7B gene, when both the proband (initial affected individual), and both parents' DNA is available to test.
It is important to acknowledge the limitations of the genetic testing technology when it comes to diagnosing Wilson Disease and carrier status. While sequencing the entire ATP7B gene is likely to find the majority of variants, it is also possible for disease-causing changes in the ATP7B gene to be found in regulatory elements like the UTRs (untranslated regions) and promoters, as well as in splice sites, which may be more difficult to find.
Resources
For more information on Wilson disease and access other resources, visit:
- Wilson Disease Association: http://www.wilsonsdisease.org/
- Canadian Liver Foundation: https://www.liver.ca/patients-caregivers/liver-diseases/wilson-disease/
- American Liver Foundation: https://liverfoundation.org/for-patients/about-the-liver/diseases-of-the-liver/wilson-disease/
- National Organization for Rare Disorders: http://rarediseases.org/rare-diseases/wilson-disease/
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 European Association for the Study of the Liver (2012). EASL Clinical Practice Guidelines: Wilson’s disease, Journal of Hepatology, 56(3), 671-685
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Roberts, EA & Schilsky, ML (2008) Diagnosis and Treatment of Wilson Disease:An Update. Hepatology, 47(6),2089-2111
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 [1], Weiss KH. Wilson Disease. 1999 Oct 22 [Updated 2013 May 16]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016.
- ↑ 4.0 4.1 Kumar, S., Kurian, G., Eapen, C.E., & Roberts, E. (2012). "Genetics of Wilson's Disease: a clinical perspective". Indian Journal of Gastroenterology. 31(6): 285–293.CS1 maint: multiple names: authors list (link)
- ↑ 5.0 5.1 5.2 Kitzberger, R, Madl, C & Ferenci, P. (2005) Wilson disease. Metab Brain Dis, 20(4):295-302.
- ↑ Ferenci, Peter (2017). "Chapter 14 - Diagnosis of Wilson disease". In Członkowska, Anna; Schilsky, Michael L. (eds.). Handbook of Clinical Neurology. Elsevier. pp. 171–180.
- ↑ Langwińska-Wośko, E., Litwin, T; et al. (2016). "The sunflower cataract in Wilson's Disease: a pathognomonic sign or rare finding?". Acta Neurologica Belgica. 116: 325–328. Explicit use of et al. in:
|last=(help)CS1 maint: multiple names: authors list (link) - ↑ [2], National Library of Medicine (US). Genetics Home Reference [Internet]. Bethesda (MD): The Library; 2016 Mar 9. Wilson disease; [reviewed 2014 Aug; cited 2016 Mar 9]. Available from: https://ghr.nlm.nih.gov/condition/wilson-disease
- ↑ 9.0 9.1 9.2 Ferenci, P (2017). "Diagnosis of wilson disease". Handbook of Clinical Neurology. 142: 171–180 – via Elsevier Health Sciences.
- ↑ Merle, Uta, Wolfgang Stremmel, and Jens Encke. "Perspectives for gene therapy of Wilson disease." Current gene therapy 7.3 (2007): 217-220.