Course:MEDG550/Student Activities/Beckwith-Wiedemann Syndrome
Beckwith-Wiedemann Syndrome (BWS) is a genetic disorder present from birth that affects the growth of many parts of the body. It is caused by the presence of too much growth signal, resulting in overgrowth of certain body parts, an increased risk for tumour development, and several different birth anomalies.
Clinical Characteristics
BWS is thought of as a clinical spectrum. This means that affected individuals can have many features, or only one or two.[1]
Common Features[1][2]
- Larger than average in infancy (macrosomia)
- Only one side of the body growing to be abnormally large, resulting in an uneven appearance (hemihyperplasia)
- Large tongue (macroglossia)
- Born with an opening in the wall of the stomach near the belly button that abdominal organs can protrude through (omphalocele)
- Enlarged organs (viscromegaly)
- Changes in the appearance of the ears, either creases near the ear lobes or small holes known as ear pits
- Tumor development in childhood
- Abnormalities of the kidneys
Less Common Features[2]
- Low blood sugar during the newborn period (neonatal hypoglycaemia)
- Changes in the pigment of the skin on the face or back of the neck appearing as red spots (vascular lesion including nevus simplex or hemangioma)
- A characteristic appearance to the face
- Enlargment or structural changes of the heart (cardiac anomalies or cardiomegaly)
- Splitting of the abdominal muscles (diastasic recti)
- Advanced bone age
- Extra amniotic fluid during pregnancy (polyhydraminos)
Adulthood[3]
Even though infants with BWS are considerably larger than normal (macrosomia) and will continue to be larger than their peers in childhood, adults with BWS are often not unusually tall. Growth begins to slow down around the time a child is eight years old. In addition, the unique growth pattern known as hemihyperplasia, where one side of the body appears larger than the other, may become less apparent over time.
Some of the most common health issues adults with BWS face are persistent speech and swallowing difficulties associated with having an enlarged tongue in childhood (macroglossia), scoliosis, thought to come about as a result of having limbs that are different sizes and infertility in males. Overall most children and adults with BWS will not have serious medical problems associated with their condition, their life expectancy is usually normal.
Diagnosis[1]
There is no set number of features that an individual must present with in order to be diagnosed with BWS. BWS represents a clinical spectrum so some individuals will be more severely affected than others. In general, BWS should be suspected if individuals have one or more of the above listed features.
Another way to confirm a diagnosis of BWS is through genetic testing. There are several different types of genetic testing approaches. Each strategy is tailored to detect a particular genetic change known to cause BWS. The different types of molecular changes are listed under the Inheritance section on this page. Genetic testing can identify a particular causative alteration more than 80% of the time. This does however mean that a genetic cause will not be identified in 20% of individuals with BWS.
Management
Treatment and management of BWS depends on what features an individual presents with. Because individuals with BWS can differ greatly from one another in their clinical features, some people with BWS will require a lot more medical interventions and follow-up than others. Listed below are some of the more common features that might require some sort of medical intervention or management.[1]

Macroglossia: The tongue of some individuals may be large enough that it can cause breathing, speech or feeding difficulties. Breathing issues might also result in sleep apnea. The child's ability to breathe should be assessed and monitored. Some individuals may wish to have a sleep study to help address the concern of sleep apnea. Feeding difficulties can be managed by using specialized nipples that attach to a babies bottle or, if severe enough, the short-term use of a feeding tube inserted through the babies nose. Speech difficulties can be addressed by follow-up with a speech pathologist. Some children may even benefit from tongue reduction surgery.
Omphalocele: Omphalocele is often detected prior to birth on ultrasound. Omphalocele will require a surgery soon after birth to repair the abdominal wall.
Cardiac Problems: Different changes in either the structure of the heart or its ability to work properly may require surgery after birth. This intervention depends a lot on what the actual defect is and how it is affecting the hearts ability to pump blood.
Low Blood Sugar: It is very important that blood sugar is monitored and treated. Low blood sugar (hypoglycemia) in newborns can cause damage to the central nervous system. Sometimes the hypoglycaemia doesn't set in right away, so it is important for children to be monitored and parents informed of the symptoms of hypoglycaemia so that they can seek appropriate medical attention. If a baby is found to have low blood sugar, it is given a source of sugar (glucose) that acts quickly to raise sugar levels in the blood.
BWS and the risk of tumor development
Children with BWS have a higher chance of developing tumors early in their life. Most tumors occur before the age of 4. It is very rare that a tumor associated with BWS would develop after the first decade of life. The percentage of children with BWS who develop a tumour is around 10%, meaning that 90% of children with BWS do not develop any tumors. The most common tumor is a growth in the kidney known as a Wilms tumor. Other tumours can be observed as well. Tumor risk varies based on the specific molecular or genetic change that caused a person to develop features associated with BWS. Some causes of BWS have a much higher associated tumor risk, while others are much lower.[4] Because of the increased risk for tumor development, periodic screening during childhood (of the kidneys in particular) is recommended.[1]
Male Infertility
The association between male infertility and BWS is not well understood, but it has been documented that some males affected with the condition have been unable to reproduce. Because of this association, it is recommended that adult males be offered testing to assess their fertility status.[3]
Inheritance

Humans normally have 46 chromosomes that are made up of our genetic information or DNA. Chromosomes come in pairs, so we each typically have two copies of each chromosome. We get half of our chromosomes from each parent; 23 from the egg and 23 from the sperm
Individuals with BWS have changes in a specific region of chromosome 11. These changes occur in the 11p15.5 region of the chromosome and prevent one or more genes from functioning properly. The 11p15.5 region contains the following genes that can be involved in BWS: CDKN1C, H19, IGF2, KCNQ1, and KCNQ1OT1. These genes are involved in cell growth and are activated (expressed) or inactivated (silenced) depending on whether they are found on the chromosome inherited from the egg or the sperm. A gene’s activity is controlled by methylation. Methylation is a special mark added to DNA that can be thought of as a switch controlling whether a gene is turned “on” or turned “off”. If there is methylation, the switch is turned “on” and the gene is active. If there is no methylation, the switch is turned “off” and the gene is inactive. Areas called imprinting regions are involved in controlling methylation and which nearby genes are turned "on" or "off".
BWS is an overgrowth condition, resulting from there being too much growth signal produced. Normally, genes in the 11p15.5 region inherited from the egg have a methylation pattern that signals "don't grow" and genes from the sperm signal "grow" to their cells. The balance between having "on" and "off" switches in the right location is what ensures a baby grows at a normal rate.[5]
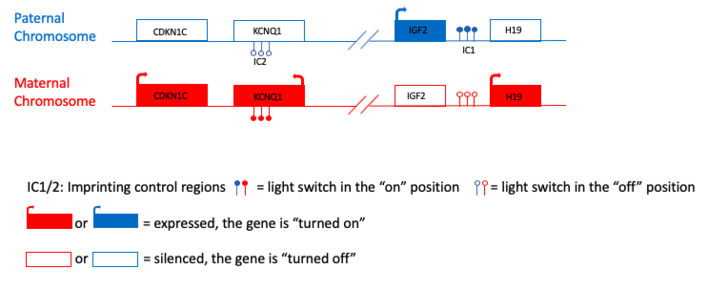
BWS can be caused by several different changes that result in there not being the right balance between "on" and "off" switches. In 80% of individuals with BWS, one of the following five changes can be detected:[6]
- Loss of methylation of IC2 on the maternal chromosome: A loss of methylation at imprinting center 2 (IC2) can be thought of as turning the switch "off". The genes CDKN1C and KCNQ1 are inactive. In this situation, mom's "don't grow" signal has been turned off.
- Gain of methylation on IC1 on the maternal chromosome: A gain of methylation at imprinting center 1 (IC1) can be thought of as turning the switch "on". The gene IGF2 is active. IGF2 produces a "grow" signal that would normally be turned off when it is inherited from mom.
- Paternal uniparental disomy: This occurs when an error is made during cell division that results in a child inheriting two copies of chromosome 11 or the 11p15.5 region from their dad and no copies from their mom. An individual with two copies of the 11p15.5 region on chromosome 11 from dad will have too much "grow" signal.
- Disease-causing variant on the maternal copy of CDKN1C: Variants or spelling mistakes in the CDKN1C gene can prevent the gene from being able to work properly. These changes can result in mom's "don't grow" signal being turned off, but only when the spelling mistake is inherited from mom.
- 11p15.5 duplication, inversion, or translocation: Other types of genetic changes where all or part of the 11p15.5 region gets copied (duplicated), flipped around (inverted), or moved (translocated) can also result in there not being an imbalance between "grow" and "don't grow” signals.
For a visual representation of the light switch metaphor. https://media.chop.edu/data/files/pdfs/bws-coloring-book.pdf
Prevalence and Population Frequencies
BWS is the most common overgrowth syndrome, it has a incidence of approximately 1 in 13,700 births.[7] However, this number likely underestimates the number of individuals with BWS considering the fact that individuals with milder phenotypes might be undiagnosed.[1]
Recurrence Risk[1]
Approximately 85% of BWS cases are sporadic. This means that the genetic change that caused the syndrome happened for the first time in this person. The remaining 15% are familial cases, meaning an affected individual inherited the genetic change from one of their parents. The majority of families have a recurrence risk (the chance a couple might have another child affected with BWS) of less than 1%. However, certain genetic changes that cause BWS can have a much higher recurrence risk, as high as 50%. This is another reason why it might be important to identify an underlying genetic cause. Not only does it confirm the diagnosis of BWS but it can also allows families to have a more accurate sense of their recurrence risk.
Genetic Counselling
Reason for Referral:
Someone may be referred to a genetic counsellor to discuss BWS for several reasons. Some reasons may include[8]:
- they may be pregnant and have signs of BWS observed on an ultrasound
- they may have a child with one or more clinical features of BWS
- they may have a family history of BWS, or they have BWS themselves, and are wondering about the possibility of passing this on to their children
With any of these patients, a genetic counsellor may discuss the inheritance pattern, the recurrence risk, and options and considerations for genetic testing[8]. During the appointment, genetic counsellors will also aim to address any concerns or issues that the patient/family is encountering with respect to their symptoms or diagnosis. They will provide support and and resources/referrals to address the patient/family's concerns.
Genetic Testing:
If a patient shows some clinical features of BWS, they may be offered genetic testing, which could provide them with a diagnosis and an idea of the recurrence risk and some associated clinical features that they might expect to find with their specific genetic cause of BWS[9]. Typically, if BWS is suspected, genetic testing may begin with DNA methylation testing to see which genes are turned "off" or "on"[9]. If this test is positive, further testing may be pursued to see if it is a loss or gain of methylation, or potentially uniparental disomy (refer to Inheritance section for a review of these terms). Further testing may involve testing of the maternal and paternal DNA, if possible, in order to differentiate which genetic material has come from either the egg or the sperm[9]. Another testing option that may be offered looks for variants in the CDKN1C gene, which may lead to it not functioning properly. After this, whole-exome or whole-genome sequencing may be an option, which involves searching the patient's DNA for any variants, or spelling mistakes, that could potentially cause the clinical features that are observed. This journey that a family or individual may embark on to search for a diagnosis of their symptoms is often referred to as the "diagnostic odyssey"[10]. This time can feel very confusing, frustrating, and hopeless for families as they move back and forth between specialists without obtaining a diagnosis[11]. It is important for genetic counsellors to be mindful of this and to allow the patients to voice their concerns, while staying open with them about the limitations of genetic testing.
Prenatal Genetic Testing:

Prenatal genetic testing may be offered when BWS is suspected in a pregnancy due to either a family history of BWS or, more, commonly, ultrasound findings like omphalocele or increased growth[8]. Prenatal genetic testing may be offered in order to further clarify the reason behind these findings or to make preparations for delivery which may include a recommendation for a hospital delivery, monitoring the baby for hypoglycemia, and surveillance for tumors[8]. In some cases, a positive test result may lead to the decision to terminate the pregnancy[8]. One prenatal testing option is Chorionic Villus Sampling. This options involves the aspiration of a small sample of the placenta. The placenta will most likely carry the same DNA as the fetus, so this placental DNA is then tested for some genetic conditions. This option would be offered from 11-13 weeks in the pregnancy[12]. Another option is called amniocentesis. This option is offered at 15-18 weeks in the pregnancy and involves using a needle to poke through the abdomen to obtain a small sample of amniotic fluid. This fluid, which contains some of the fetus' skin cells, will be tested for some genetic conditions[12]. With both of these options, there is a risk for loss of the pregnancy ranging from 0.5-2%. Families should be counselled about their reason for pursuing this testing and their subsequent options in order to determine if it is right for them[8][12].
Psychosocial Considerations:
It is important to remember that BWS has a variable spectrum of clinical presentations which cannot always be predicted during the prenatal period or even during infancy[8]. It can be very difficult to learn that your child may have a genetic disorder, and it may take some time for parents to reframe their idea of who their child might be. Parents may struggle with managing their expectations, especially with the uncertainty and wide variability in the clinical presentations of BWS[8]. The time and money that may be required to manage the child's symptoms can also be a concern to parents and may affect some more than others based on their profession, income, and lifestyle.
Patient Resources
Beckwith-Wiedemann Children's Foundation International
https://www.beckwithwiedemann.org
Beckwith Wiedemann Support Group
Beckwith-Wiedemann Coloring Book: Developed through conversations with families of children with Beckwith-Wiedemann Syndrome (BWS), this coloring book includes simple illustrations and easy-to-understand descriptions of the genetic and epigenetic causes of BWS, as well as information on managing BWS.
https://media.chop.edu/data/files/pdfs/bws-coloring-book.pdf
To locate a genetics clinic near you, please visit www.cagc-accg.ca (Canada) or www.nsgc.org (United States).
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Shuman C, Beckwith JB, Weksberg R. (2016). "Beckwith-Wiedemann Syndrome". GeneReviews.CS1 maint: multiple names: authors list (link)
- ↑ 2.0 2.1 Choufani S, Shuman C, Weksberg R (2010). "Beckwith-Wiedemann syndrome". American Journal of Medical Genetics. 153: 343–354.CS1 maint: multiple names: authors list (link)
- ↑ 3.0 3.1 Gazzin A, Carli D, Sirchia F; et al. (2019). "Phenotype evolution and health issues of adults with Beckwith-Wiedemann syndrome". American Journal of Medical Genetics. 179: 1691–1702. Explicit use of et al. in:
|last=(help)CS1 maint: multiple names: authors list (link) - ↑ Rump P, Zeegers MPA, van Essen AJ (2005). "Tumor risk in Beckwith-Wiedemann syndrome: A review and meta-analysis". American Journal of Medical Genetics. 136A: 95–104.CS1 maint: multiple names: authors list (link)
- ↑ The Children's Hospital of Philadelphia (2016). "BWS and You: An Educational Coloring Book". Retrieved January 13th 2020. Check date values in:
|access-date=(help) - ↑ Choufani S, Shuman C, Weksberg R (2013). "Molecular Findings in Beckwith-Wiedemann Syndrome". American Journal of Medical Genetics. 163: 131–140.CS1 maint: multiple names: authors list (link)
- ↑ Engstrom W, Lindham S, Schofield P (1988). "Wiedemann-beckwith syndrome". European Journal of Pediatrics. 147: 450–457.CS1 maint: multiple names: authors list (link)
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 Eggermann T, Brioude F, Russo S, Lombardi MP, Bliek J, Maher ER, Larizza L, Prawitt D, Netchine I, Gonzales M, Grønskov K, Tümer Z, Monk D, Mannens M, Chrzanowska K, Walasek MK, Begemann M, Soellner L, Eggermann K, Tenorio J, Nevado J, Moore GE, Mackay DJ, Temple K, Gillessen-Kaesbach G, Ogata T, Weksberg R, Algar E, Lapunzina P. Prenatal molecular testing for Beckwith-Wiedemann and Silver-Russell syndromes: a challenge for molecular analysis and genetic counseling. Eur J Hum Genet. 2016 Jun;24(6):784-93. doi: 10.1038/ejhg.2015.224. Epub 2015 Oct 28. PMID: 26508573; PMCID: PMC4867462.
- ↑ 9.0 9.1 9.2 Brioude F, Kalish JM, Mussa A, Foster AC, Bliek J, Ferrero GB, Boonen SE, Cole T, Baker R, Bertoletti M, Cocchi G, Coze C, De Pellegrin M, Hussain K, Ibrahim A, Kilby MD, Krajewska-Walasek M, Kratz CP, Ladusans EJ, Lapunzina P, Le Bouc Y, Maas SM, Macdonald F, Õunap K, Peruzzi L, Rossignol S, Russo S, Shipster C, Skórka A, Tatton-Brown K, Tenorio J, Tortora C, Grønskov K, Netchine I, Hennekam RC, Prawitt D, Tümer Z, Eggermann T, Mackay DJG, Riccio A, Maher ER. Expert consensus document: Clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: an international consensus statement. Nat Rev Endocrinol. 2018 Apr;14(4):229-249. doi: 10.1038/nrendo.2017.166. Epub 2018 Jan 29. PMID: 29377879; PMCID: PMC6022848.
- ↑ Davlin AS, Clarkin CM, Kalish JM. Beckwith-Wiedemann Syndrome: Partnership in the Diagnostic Journey of a Rare Disorder. Pediatrics. 2018 Mar;141(3):e20170475. doi: 10.1542/peds.2017-0475. Epub 2018 Feb 2. PMID: 29437884; PMCID: PMC5847091.
- ↑ Miller D. The diagnostic odyssey: our family's story. Am J Hum Genet. 2021 Feb 4;108(2):217-218. doi: 10.1016/j.ajhg.2021.01.003. PMID: 33545028; PMCID: PMC8175868.
- ↑ 12.0 12.1 12.2 Nussbaum, R. L., McInnes, R. R., & Willard, H. F. (2015). Thompson & thompson genetics in medicine e-book. Elsevier.