Course:MEDG550/Student Activities/ARVC

Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited disease of the heart muscle that can sometimes cause dangerous heart rhythms. These irregular heart rhythms (also known as arrhythmias) are due to weakened connections between the heart muscle cells. When these connections are weak, deposits of fat and scarring can build up in the muscle tissue. This usually happens in the right ventricle (the lower chamber on the right side of the heart). This scarring can then affect the heart’s electrical circuit.[1]
ARVC can also referred to as ARVD, or arrhythmogenic right ventricular dysplasia.
Clinical Characteristics
ARVC is a disorder of the muscular wall of the heart (called the myocardium). This condition causes parts of the muscle to be replaced with fatty deposits and scar tissue, usually in the right ventricle but occasionally in the left ventricle as well.[2]
The presentation of ARVC is highly variable, even within families. Many people with ARVC will never develop signs or symptoms of the condition.[3] The age of onset is also quite variable, ranging from adolescence to late adulthood.
Initial symptoms of ARVC can include:
- Racing, skipping or fluttering sensations in the chest (palpitations)
- Light-headedness
- Unexplained fainting (syncope)
Over time, ARVC can also cause shortness of breath and swelling in the legs or abdominal region. If the muscle becomes severely damaged in the later stages of the condition, this can progress to heart failure.[4] In rare cases, sudden cardiac death may be the first presentation of the condition.
Diagnosis
ARVC is a complex condition to diagnose. Currently, there is a checklist of criteria used.[5][6] Individuals may be classified as having definite, borderline or possible diagnosis of ARVC based on the number of major/minor criteria present. This is evaluated by family history as well as a variety of tests:
- Echocardiogram – Ultrasound images of the heart’s structure and function can identify the fatty deposits and scarring of the cardiac muscle and may show other signs of the condition’s progression.
- MRI – This test can give a more detailed picture of the cardiac muscle than the echocardiogram. MRIs are not available at every centre, and there may be reasons why someone should not get an MRI (such as having certain types of cochlear implant or pacemaker, or having artificial joints or limbs). Talk to your doctor or radiologist if you know that you have any metal equipment implanted in your body.
- Electrocardiogram (ECG) – A test which measures and records the signal of the electrical circuit in the heart. Individuals with ARVC will have specific patterns that are noticeable by a cardiologist.
- Holter Monitor – A device which continuously records your heart’s rhythm for a longer time period than the ECG, typically for 24 or 48 hours. This is most useful when an individual has changes for short periods throughout the day or with certain triggers that may not be picked up by an ECG.
- Exercise Stress Test – Many people only have changes in their heart’s rhythm during physical exertion. This test records the heart’s electrical response to activity when the heart is beating harder and faster than it does at a resting state.
- Genetic Testing – This is commonly used to confirm a diagnosis and can provide a better picture as to the most effective management strategies. Finding a causal variant may provide the opportunity for cascade testing in family members to identify others who may be at risk.
- Autopsy – Unfortunately, sometimes ARVC is only diagnosed after an individual has passed away unexpectedly. Autopsy results may show abnormalities in the heart muscle, and in this case family members should be subsequently seen by a cardiologist.
Management
Although there is currently no cure for ARVC, there are things that people with ARVC can do to reduce the effects of this condition. This will depend on factors that are specific to you and your heart, and your doctor will be able to determine which treatment option may work best based on your specific case.[7]
- Surveillance (“watch and wait”) – Your doctor may choose to repeat your tests over time to watch for progression of the condition before taking any further steps.
- Medications can improve the heart muscle function or control abnormal heart rhythms, such as beta-blockers (which help lower the heart rate, blood pressure and effects of adrenaline).
- Implantable cardioverter-defibrillator (ICD) is a device that is implanted in the body to monitor the heart’s rhythm and can deliver small electrical shocks to the heart if someone experiences a dangerous heart rhythm. Not all patients with ARVC will need an ICD.
- Exercise restriction is recommended, as exercise can speed up the progression of the disease. Choosing forms of activity that put less strain on the heart is important. Your doctor can help you find activities that are safe and healthy for your heart condition.
- Heart transplantation may be suggested in rare cases if the heart muscle becomes very weak.
Genetics
In most cases, ARVC is caused by a change (often called a variant) in a gene. A gene is a piece of genetic information (DNA) that encodes for a specific instruction or job within our body.
There are some genes responsible for maintaining the cardiac muscle, particularly the proteins that hold the cells together called the desmosome. If we have a change in one of the genes involved in the desmosome, this can result in ARVC. We currently know of about seven of these genes, and changes in these genes have been found in 40-60% of individuals with ARVC.
| Gene (Symbol) | Prevalence[8] |
|---|---|
| Plakiphili-2 (PKP2) | 34-74% |
| Desmoplakin (DSP) | 2-39% |
| Desmoglein-2 (DSG2) | 5-26% |
| Desmocollin-2 (DSC2) | 1-2% |
| Plakoglobin (JUP) | 0.5-2% |
| Transmembrane protein 43 (TMEM43) | Rare |
| Cardiac Ryanodine Receptor (RYR2) | Rare |
Not everyone with an ARVC-associated variant ends up developing symptoms of the condition. It is thought that there are other factors that play into whether someone will develop the condition such as other genes as well as the environment, but these factors are not yet well understood.
Prevalence and Population Frequencies
The prevalence of ARVC is estimated to be 1/2000 to 1/5000 in the general population, and men are affected three times (3X) as often as women.[9] In certain regions (such as parts of Italy and Greece) the prevalence is much higher.
Inheritance

Autosomal Dominant
Humans have two copies of each of our genes—we inherit one copy of from our mother and the other from our father. ARVC is usually inherited in an autosomal dominant manner, meaning that a person only needs a change in one copy of an ARVC-associated gene to be at risk for the condition. This change could have been inherited from either parent or may have occurred randomly during fertilization.
If a person has a change in one of these genes, there is a 50% chance of passing that on to each of their children. It is recommended that all first-degree relatives (children, parents and siblings) receive cardiac screening for this condition, and they may also be eligible for genetic testing.
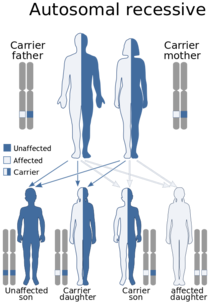
Autosomal Recessive
In an unusual form of ARVC (called Naxos disease), the condition can be autosomal recessive. Naxos disease is also known as palmoplantar keratosis and is associated with woolly hair and various skin changes. It is seen largely in Naxos Island, Greece.
Autosomal recessive means that to develop the condition, both copies the gene must have changes which cause them not to work properly. If only one copy of the gene has this change, and the other copy is normal, the individual is a carrier of the condition. Carriers often normal heart function and typically don’t ever realize they are a carrier. Sometimes, people with one copy of the gene change will have slightly woolly hair.
Genetic Testing
There are tests that look for changes in genes that have been associated with ARVC or related inherited heart muscle diseases. There are three possible results from this type of genetic test:
- A positive result means a change was found in one of these genes. Approximately 50% of ARVC patients will receive this result. A positive genetic result can help confirm the diagnosis of ARVC. Also, genetic testing can typically be offered to close family members to help determine who is at an increased risk of developing this condition.
- A negative result means no changes were found in any of these genes. A negative result does not rule out the diagnosis. Genetic testing would not be available for family members. The cause of ARVC could still be inherited but our current test is not able to identify the specific genetic cause. It is still important for close family members to have heart screening tests. Many of these cases are due to changes in genes that we are not aware of yet, but research continues to be done.
- A variant of uncertain significance (VUS) means that a change was found, but it is unknown at this time whether this is the cause of ARVC. Patients with a VUS should continue to have regular cardiac screening, and close family members should also be seen by a cardiologist for assessment.
Patient Resources
Sudden Arrhythmia Death Syndromes (SADS) Foundation
American Heart Association (AHA)
To locate a genetics clinic near you, please visit www.cagc-accg.ca (Canada) or www.nsgc.org (United States).
References
- ↑ Burke, A.P., Farb, A., Tashko, G., Virmani, R. (1998). "Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: Are they different diseases?". Circulation. 97: 1571–1580.CS1 maint: multiple names: authors list (link)
- ↑ Hamid, M.S; et al. (2002). "Prospective evaluation of relatives for familial arrhythmogenic right ventricular cardiomyopathy/dysplasia reveals a need to broaden diagnostic criteria". Journal of the American College of Cardiology. 40: 1445–1450. Explicit use of et al. in:
|last=(help) - ↑ Groeneweg, J.A.; et al. (2015). "Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members". Circulation. Cardiovascular Genetics. 8: 437–446. Explicit use of et al. in:
|first=(help) - ↑ Bhonsale, A.; et al. (2015). "Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers". European Heart Journal. 36: 847–855. Explicit use of et al. in:
|last=(help) - ↑ McKenna, W., Thiene, G. & Nava, A. (1994). "Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy". British Heart Journal. 71: 215–218.CS1 maint: multiple names: authors list (link)
- ↑ Marcus, F.I.; et al. (2010). "Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria". European Heart Journal. 31: 806–814. Explicit use of et al. in:
|last=(help) - ↑ Corrado, D.; et al. (2015). "Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement". European Heart Journal. 36: 3227–3237. Explicit use of et al. in:
|last=(help) - ↑ McNally, E., MacLeod, H., and Dellefave-Castillo, L. "Arrhythmogenic Right Ventricular Cardiomyopathy". GeneReviews.CS1 maint: multiple names: authors list (link)
- ↑ Patel, D.M. & Green, K.J. (2014). "Desmosomes in the Heart: A Review of Clinical and Mechanistic Analyses". Cell Communication and Adhesion. 21: 109–128.CS1 maint: multiple names: authors list (link)