Course:FNH200/Projects/2022/Infant Formula
Introduction

Infant formula is an invention that tries to simulate breast milk as close as possible. Infant formula was originated from the necessity to find an alternative when there was an inability to breast-feed. Although being introduced almost 4000 years ago, it would take until the end of the 20th century to reach the modern infant formula that we know today. Infant formula is created with various formulation as close to human milk as possible to meet the nutritional demand that infants require.
Origin, Usage and Importance
History

Prior to the invention of infant formula, wet-nurses were often employed to provide infants with nutrition if the birth mother was unable to nurse their baby. If a wet-nurse was not available, or if the baby required more nutrition than the mother could provide, additional substitutes with another species of animal’s milk, predigested food or wheat-containing porridges were sometimes used. Until the invention of the modern feeding nipple and the development of properly sterilized and appropriately designed infant formula, infant mortality rates were high in babies who were fed breast milk alternatives. A brief timeline is shown in the appendix section.
Usage of infant formula
There are various reasons why exclusively breastfeeding may not be possible: these include inadequate supply, adopted parents, socio-cultural demands on the mother, or certain health conditions in the mother or child demanding complete replacement or partial supplementation with another food source. A significant benefit of non-breast milk feeds is that any caregiver can feed the baby – greatly reducing strain to the mother. A 2017-2018 survey found that 91% of Canadian mothers initiate breastfeeding, but only 34% continue exclusive breastfeeding for 6 months[2]. Although human breast milk is the gold standard of infant food, there are situations where formula is required and appropriate: infant formula is a critical tool in assuring the health and safety of babies around the world.
Importance of formula
Infant formula is a dairy substitute that aims to synthetically recreate human milk – a significant challenge given the complexity of breastmilk. Breastmilk is very dynamic, fundamentally tailor-made by each mother for her baby: it changes from mother to mother, and feed to feed, depending on factors such as the time of day, age of the baby, diet of the mother, and what diseases she has been exposed to[3]. The uniqueness of breastmilk is why breastfed babies are thought to be generally healthier than formula fed babies. While it is impossible to recreate the intra- and inter- variation, infant formula attempts to appropriately replace the nutrition provided by human milk by blending fat, protein and carbohydrates and including a wide range of supplements, vitamins, minerals and other compounds. The requirements are so specific that it can be considered a hybrid of a food and pharmaceutical product[1].
Formulation


As the only food that babies may receive in their first six months of life, it is critical that infant formula mimics human milk in terms of macro and micronutrients, is sterile, and is the right texture and taste to be appealing[1][4]. Formula is governed by Division 25 of the Food and Drug Regulation which pertains to “Human Milk Substitutes and Foods Containing Human Breastmilk”[4]. Importantly, B.25.060 (4)a. defines that: “[it must be] established that the human milk substitute is nutritionally adequate to promote acceptable growth and development in infants when consumed in accordance with the directions for use[4].” Fat is an important component of breastmilk as it provides 50% of the energy required and the fatty acid profile is unique to our species and critical for human development[1]. While infant formula provides adequate nutrition to babies, it is currently not possible to mimic breastmilk’s dynamic nature as there are more than 200 human milk oligosaccharides (sugars), as well as specific digestive enzymes, hormones and immune cells, which are too complex to artificially recreate[5][6].
| Comparison of milk products | |||
|---|---|---|---|
| Breast Milk |
| ||
| Infant Formula |
|
Hypoallergenic Formula |
|
| Hydrolyzed Formula |
| ||
| Extensively Hydrolyzed Formula |
| ||
| Free Amino-acid based Formula |
| ||
The most common infant formulas start with a cow milk base, which has higher levels of fat, minerals and proteins than human milk so it must be skimmed and diluted. An assortment of oils are then added to mimic the fatty acid profile of human milk, along with minerals, vitamins and other compounds. The ratio of milk proteins must also be manipulated as cows milk contains a much higher ratio of casein to whey than human milk[3][5].

| Component | Cow's Milk | Human Milk |
|---|---|---|
| Lactose (%) | 4.5 | 7.0 |
| Protein (%) | 3.4 | 1.0 |
| Casein (%) | 2.6 | 0.3 |
| Whey Protein (%) | 0.8 | 0.7 |
| Whey Protein : Casein (Ratio) | 20 : 80 | 70 : 30 |
| Fat (%) | 4.0 | 3.8 |
| Mineral (%) | 0.7 | 0.2 |
There are three classes of infant formula: (1) Cow-milk based is the most common. (2) Soy-based for babies who are allergic to cow milk (2-7% of all babies), or are culturally unable to drink cow milk, and (3) Specialized, for example a hypoallergenic formula, consisting of hydrolyzed proteins, for babies who can’t tolerate cow or soy-based formulas[3][5]. While all the formulas have the same bases (carbohydrate, protein, fatty acids, amino acids, nucleic acids and preservatives) there is a wide variety in terms of the types of fats and amino acids added – this can likely be explained by an attempt by the manufacture to fill a void of critical nutrients not present in the base.

Formula also comes in three forms: (1) Powder, (2) Liquid concentrate and (3) Ready-to-feed (the most expensive). While ready-to-feed is prediluted, sterilized, and individually packaged, powder and liquid concentrate must be diluted – this can pose a food safety risk if the mixing water is contaminated. It is recommended that preterm or low-birth weight infants only be fed ready-to-feed formula or liquid concentrate that has been re-diluted with boiled and cooled water[4][5].
Processing
Production & Dehydration Practices in the industry
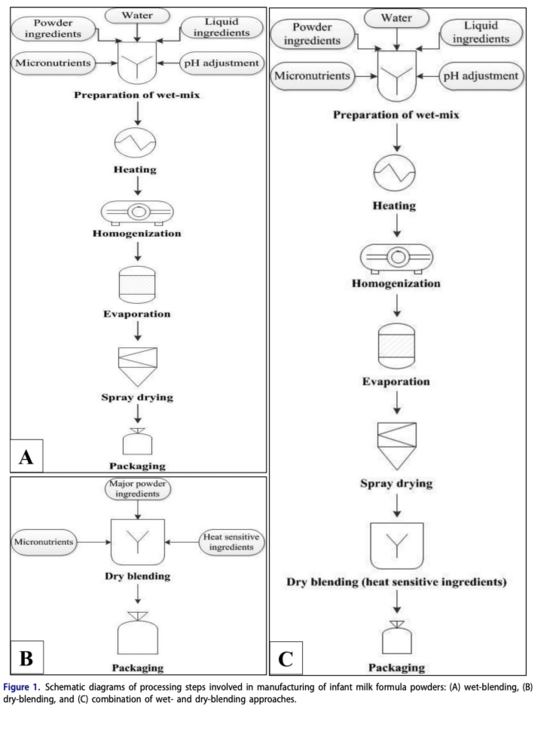
Typical powdered infant formula is commonly produced in one of two ways: wet-blending and dry-blending[7]. In wet-blending, a wet concentrate containing all ingredients is spray-dried to produce IMF powders. In dry-blending, all ingredients are in powder form, and are then dry-blended to attain the desired composition[7]. Although the industry uses both of these methods, wet-blending is more prominent as it can be controlled much more easily and can subsequently produce formulas with better physicochemical & microbial properties[7].
An important stage to highlight is homogenization as it stabilizes the emulsions in the formula. Another critical step that comes after is pasteurization (not shown on diagram), used in order to kill the pathogenic microorganisms. The powdered formula will then be canned in aseptic packaging and is packed under modified atmosphere such as nitrogen, to prevent the product from oxidizing and thereby extending the shelf life[1]. It should be noted that sterilization and subsequent aseptic packaging into single-shot sachets is required for liquid ready to eat formulas[1].
 |
 |
|---|
Formula Regulation & Safety in Canada
There are necessary steps to be taken before a formula is allowed to be put onto the market and advertised, this requires informing the minister with a minimum of 90 days prior to being put onto the market. Other aspects of the infant formula must also be included, such as:
- List of all of ingredients, stated quantitatively[4]
- Specifications for nutrient, microbiological & physical quality for ingredient & new human milk substitute
- Details of quality control procedures that respect testing of ingredients and oh new milk substitute
- Details of manufacturing process & quality control procedures throughout the process
- Evidence to establish that the new human milk substitute is nutritionally adequate to promote acceptable growth & development infants when consumed in accordance with direction usage
- Description of the type of packaging to be used
Only through approval of the stated regulations will the product be allowed to be put onto the market.
An additional layer of safety is ensured through heat treatment to sterilize formula[4]; however, powdered infant formula is not sterilized before use and thus must have instructions and safety precautions followed carefully so as to prevent foodborne illness, usually involving heating the formula to a high enough temperature to kill the microorganisms[4].
Food Storage
Breast milk is commonly frozen to store for longer periods, it can be stored at room temperature, refrigerated, stored with frozen gel packs, and frozen for varying lengths of time[8]. Once thawed, breast milk cannot be refrozen and must be used within 24 hours of being thawed[8]. It is important to realize that most of the benefit that aids to the dynamic nature for babies are highest when it is fresh, such beneficial components, like antioxidants and other protective properties decrease after refrigeration and freezing. On the same note, milk, like other liquids suffers from changes that decrease appeal when frozen; proteins can become denatured and cause the milk to curdle. Emulsions in the milk can also break down and allow fats to separate. Milk can also suffer from “freezer burn” or dehydration burn, where the milk can lose moisture. Thus, when thawing breast milk, thawed fats should be redistributed evenly so as to avoid the unappetizing changes freezing can bring[8].
Conversely, infant formula's storage is more limited, with different storage methods based on if it's a powder/concentrate or when it has been prepared.
As a dehydrated powder, unopened cans can be stored in a cool, dry place and should not be stored in the fridge. Opened cans are stored similarly as long as they have been resealed tightly with their lids[9]. The difference between unopened and resealed cans is that resealed cans should be used within 1 month of opening or before the expiration date[9], while unopened cans can remain stored and should not be used after their expiration.
Ready-to-feed and liquid concentrate formula should be stored in a cool, dry place and opened cans should be refrigerated. Both should be discarded after 48 hours in the fridge.
For both powdered and liquid concentrate formulas, if more formula is made than is needed for one feeding, then the extra should be used within 2 hours, bottles should also be cooled quickly in an ice bath or under cool running water, then refrigerated. Refrigerated rehydration powder and prepared liquid concentrate formula must be used within 24 hours due to potential for bacterial growth[8]. Like breast milk, discard any extra formula in the bottle after a feeding session as saliva from the baby may contaminate the formula with bacteria and may be detrimental to babies if ingested[8][10].
Relevancy
Infant Formula Global shortages
There is no shortage of regular milk-based formulas, though there is a shortage of hypoallergenic formulas[4]. This is because a major formula producer in the US had their factories shut down (Abbott Laboratory Manufacturing plant). This shutdown had a profound impact on Canadian as well as Global hypoallergenic formula supply. The plant was initially shut down in February due to bacterial infections in infants who consumed the formula, they reopened a while later only to have to be shut down again due to plant contamination in part due to the storms and major flooding[4].
Alternative Solutions to Formula in the Food Science Field
For the future lab grown breast milk alternatives are currently being researched. A research paper published in the Journal of Applied Science and Computations elaborates on the benefits of lab grown breast milk for mammary cells as an alternative to breast milk and formula. The article provides more information on the technique of replicating the number of mammary cells found in the mammary gland. This paper looks at why it should change the formula industry and all the benefits it will provide to infant nutrition. The expected benefits of lab grown breast milk presented in this article are that it offers the ideal nutrition for babies and possesses an almost perfect mix of vitamins, fat, and protein[11].
We would infer if all of these benefits are found in lab grown breast milk, the demand for milk based infant formula will likely decrease. This would allow for hypoallergenic hydrolyzed formulas to be the only necessary formula type hopefully ceasing shortages in the formula sector.
Appendix
Note: Information added in this section is just for extra information, Judy asked us to label extra content, which will be represented by being in the appendix section, ONLY this section will not count towards marking.

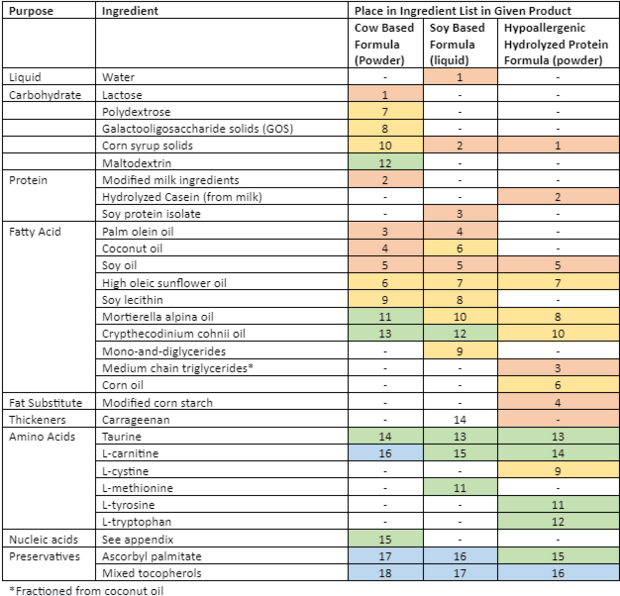
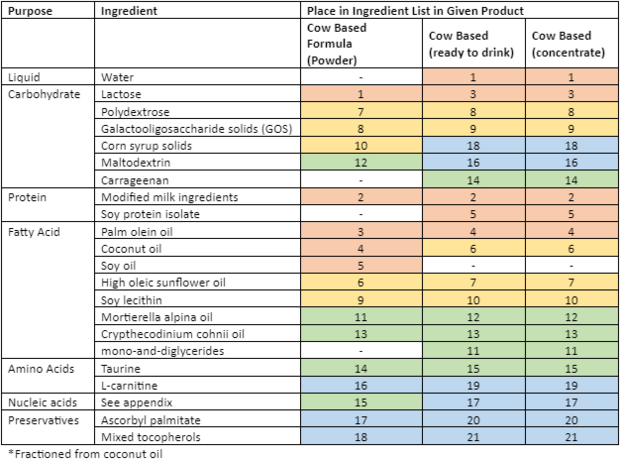
| Enfamil Cow Based Formula
(Powder) |
Enfamil Soy Based Formula (liquid) | Enfamil Hypoallergenic Hydrolyzed Protein Formula (powder) | |
|---|---|---|---|
| Brand Name | Enfamil A+ | Enfamil A+ Soy Baby Formula | Pregestimil A+ |
| Ingredients | LACTOSE, MODIFIED MILK INGREDIENTS, PALM OLEIN OIL, COCONUT OIL, SOY OIL, HIGH OLEIC SUNFLOWER OIL, POLYDEXTROSE, GALACTOOLIGOSACCHARIDE SOLIDS (GOS), SOY LECITHIN, CORN SYRUP SOLIDS, MORTIERELLA ALPINA OIL, MALTODEXTRIN, CRYPTHECODINIUM COHNII OIL*, TAURINE, NUCLEOTIDES (ADENOSINE 5'-MONOPHOSPHATE, CYTIDINE 5'-MONOPHOSPHATE, DISODIUM GUANOSINE 5'-MONOPHOSPHATE, DISODIUM URIDINE 5'-MONOPHOSPHATE), L-CARNITINE, ASCORBYL PALMITATE, MIXED TOCOPHEROLS, (MAY CONTAIN POTASSIUM HYDROXIDE) | WATER, CORN SYRUP SOLIDS, SOY PROTEIN ISOLATE, PALM OLEIN OIL, SOY OIL, COCONUT OIL, HIGH OLEIC SUNFLOWER OIL, SOY LECITHIN, MONO- AND DIGLYCERIDES, MORTIERELLA ALPINA OIL‡, L-METHIONINE, CRYPTHECODINIUM COHNII OIL§, TAURINE, CARRAGEENAN, L-CARNITINE, ASCORBYL PALMITATE, MIXED TOCOPHEROLS, (MAY CONTAIN POTASSIUM HYDROXIDE). | CORN SYRUP SOLIDS, HYDROLYZED CASEIN* (FROM MILK), MEDIUM CHAIN TRIGLYCERIDES (FRACTIONATED COCONUT OIL), MODIFIED CORN STARCH, SOY OIL, CORN OIL, HIGH OLEIC VEGETABLE OIL (SAFFLOWER AND/OR SUNFLOWER OIL), MORTIERELLA ALPINA OIL**, L-CYSTINE, CRYPTHECODINIUM COHNII OIL†, L-TYROSINE, L-TRYPTOPHAN, TAURINE, L-CARNITINE, ASCORBYL PALMITATE, MIXED TOCOPHEROLS, (MAY CONTAIN POTASSIUM HYDROXIDE) |
| Minerals | (CALCIUM CARBONATE, CALCIUM CHLORIDE, CALCIUM PHOSPHATE, CUPRIC SULFATE, FERROUS SULFATE, MAGNESIUM PHOSPHATE, MANGANESE SULFATE, POTASSIUM CITRATE, POTASSIUM IODIDE, SODIUM CHLORIDE, SODIUM CITRATE, SODIUM SELENITE AND ZINC SULFATE) | CALCIUM CARBONATE, CALCIUM PHOSPHATE, CUPRIC SULFATE, FERROUS SULFATE, MAGNESIUM CHLORIDE, MAGNESIUM PHOSPHATE, POTASSIUM CHLORIDE, POTASSIUM CITRATE, POTASSIUM IODIDE, SODIUM CHLORIDE, SODIUM SELENITE AND ZINC SULFATE | CALCIUM CARBONATE, CALCIUM CHLORIDE, CALCIUM PHOSPHATE, CUPRIC SULFATE, FERROUS SULFATE, MAGNESIUM PHOSPHATE, MANGANESE SULFATE, POTASSIUM CITRATE, POTASSIUM IODIDE, SODIUM CHLORIDE, SODIUM CITRATE, SODIUM SELENITE AND ZINC SULFATE |
| Vitamins | (ASCORBIC ACID, BIOTIN, CALCIUM D-PANTOTHENATE, CHOLINE CHLORIDE, dl-α-TOCOPHERYL ACETATE, FOLIC ACID, INOSITOL, NIACINAMIDE, PYRIDOXINE HYDROCHLORIDE, RIBOFLAVIN, SODIUM ASCORBATE, THIAMINE HYDROCHLORIDE, VITAMIN A PALMITATE, VITAMIN B12, VITAMIN D3 AND VITAMIN K1). | ASCORBIC ACID, BIOTIN, CALCIUM D-PANTOTHENATE, CHOLINE CHLORIDE, dl-α-TOCOPHERYL ACETATE, FOLIC ACID, INOSITOL, NIACINAMIDE, PYRIDOXINE HYDROCHLORIDE, RIBOFLAVIN, THIAMINE HYDROCHLORIDE, VITAMIN A PALMITATE, VITAMIN B12, VITAMIN D3 AND VITAMIN K1 | ASCORBIC ACID, BIOTIN, CALCIUM D-PANTOTHENATE, CHOLINE CHLORIDE, dl-α-TOCOPHERYL ACETATE, FOLIC ACID, INOSITOL, NIACINAMIDE, PYRIDOXINE HYDROCHLORIDE, RIBOFLAVIN, SODIUM ASCORBATE, THIAMINE HYDROCHLORIDE, VITAMIN A PALMITATE, VITAMIN B12, VITAMIN D3 AND VITAMIN K1 |
Exam Question
What is the disadvantage to powdered formulas over liquid formulas?
A. Powdered formula does not reconstitute as well to properly mimic a milk-like emulsion and therefore is not as palpable to infants.
B. Powdered formulas do not have the same nutritional value as some vitamins are damaged in the dehydration process.
C. Powdered formula is more expensive to produce than liquid formulations.
D. Powdered formula is not sterile when mixed with water and prepared and therefore is not safe to serve to low birth weight infants unless added sterilization steps are taken.
Answer: D
Explanation: As explained in the processing section of our assignment, the Canadian government emphasizes that if caregivers are providing infants with powdered formula, they must be very careful in their handling of the product.
Heat treatment is required to ensure that the formula is safe to provide to an infant, unlike liquid ready to eat infant formula. We think this is an important idea to share with all of FNH 200 as it emphasizes methods of thermal processing, which was discussed in lesson 6, while encouraging the consumer to consider these methods of thermal processing when buying products in a grocery store.
As a group we were unaware of the vast differences in safety and processing of different types of formula. We thought that highlighting this specific example might help reinforce the general concept of being aware of how our foods are processed and which products require further sterilization by the consumer.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Happe, R. P.; Gambelli, L. (2015). Infant formula. Woodhead Publishing. pp. 285–315.
- ↑ Francis, J. (2021). "Breastfeeding rates are high in a prenatal community support program targeting vulnerable women and offering enhanced postnatal lactation support: a prospective cohort study". International Journal for Equity in Health. 20: 1–13.
- ↑ 3.0 3.1 3.2 "Breastfeeding vs. Formula". Mount Sinai. 2022. Retrieved August 2022. Check date values in:
|access-date=(help) - ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 "Human milk Substitutes and Foods Containing Breast Milk Substitutes". Justice Laws Website. May 10 2022. Retrieved August 4 2022. Check date values in:
|access-date=, |date=(help) - ↑ 5.0 5.1 5.2 5.3 Martin, C. R. (2016). "Review of infant feeding: key features of breast milk and infant formula". Nutrients. 8(5): 279.
- ↑ Remmel, Adriana (January 3, 2021). "What is Infant Formula and how can Scientists make it More Like human Milk?". Chemical and Enginering News. Retrieved August 3 2022. Check date values in:
|access-date=(help) - ↑ 7.0 7.1 7.2 7.3 Masum, A. K. M.; Chandrapala, J.; Huppertz, T.; Adhikari, B.; Zisu, B. (2021). "Production and characterization of infant milk formula powders: A review". Drying Technology. 39:11: 1492–1512.
- ↑ 8.0 8.1 8.2 8.3 8.4 "Storing Breast Milk". Healthlink BC. 2022. Retrieved August 13 2022. Check date values in:
|access-date=(help) - ↑ 9.0 9.1 "Feeding Your Baby Formula: Safety Making and Storing Formula". HealthLink BC. 2021. Retrieved August 13 2022. Check date values in:
|access-date=(help) - ↑ "Infant formula preparation and storage". Centers for Disease Control and Prevention. July 13 2022. Retrieved August 13 2022. Check date values in:
|access-date=, |date=(help) - ↑ Shaji, A. G. (2020). "Lab-Grown Breast milk: The Foreseeable Future of Infant Nutrition". Journal of Applied Science and Computations. 7(8): 20–34.