LFS:SoilWeb/Interactions Among Soil Components/Adsorption of Ions
Ion adsorption and subsequent exchange are important processes that take place between soil colloidal particles (clays, organic matter, sesquioxides, and amorphous minerals) and various ions. Soil colloids serve very much as a modern bank. They are the sites within the soil where ions of essential plant nutrients are held and protected from excessive loss by leaching. Subsequently, the nutrients can be "withdrawn" from the colloidal "bank" sites and taken up by plant roots. In turn, these elements can be "deposited" or returned to the colloids through the addition of commercial fertilizers, lime, manures, and plant residues.
The charges associated with soil particles attract ions (simple and complex) of opposite charge. In soils of temperate regions, negative charges generally predominate on the soil particles (colloids), hence adsorbed cations are present in larger quantities than anions. In more highly weathered soils (e.g., in the tropics) where 1:1 type clays and Fe/Al oxides are the most dominant type of colloids, anion adsorption and exchange is relatively more prominent.
Learn more information on charges on soil colloids.
The relative strength of ion adsorption onto negatively charged soil colloids can be indicated by the lyotropic series. A portion of the lyotropic series is: Ca2+ > Mg2+ > K+ ≈ NH4+ > Na+.
The mechanistic explanation for this sequence is provided by Coulomb's Law:
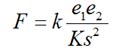
where F is the force of attraction or repulsion between two ions (N), e1 and e2 are magnitudes (or amounts) of electrical charges (C = Coulomb) on an object (or ion), s is the distance of charge separation (m), k is the proportionality constant (8.9 x 109 Nm2/C-2), and K is the dielectric constant.
Diffuse Double Layer
A double layer (DL) exists where counterions are attracted to the charged surface of a particle and form a layer. The ions are held in place by Coulombic forces. A double layer forms under very dry conditions.
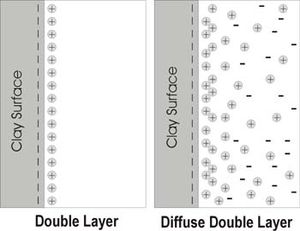
When water is added to a dry soil, the counterions are no longer held so tightly by the colloid and they begin to diffuse away from the surface. Now the electrostatic attraction between the ions in solution and the colloidal surface is counteracted by diffusion. This creates a diffuse double layer (DDL) where the charge on the particle is neutralized by a swarm of ions.
Thickness of the diffuse double layer will depend on:
- Concentration of soil solution: High concentration of soil solution yields a thin DDL
- Valence of exchange ions: Monovalent ions yield a thick DDL
- Size of an ion (or hydration radius): Strongly hydrated ions yield a thick DDL
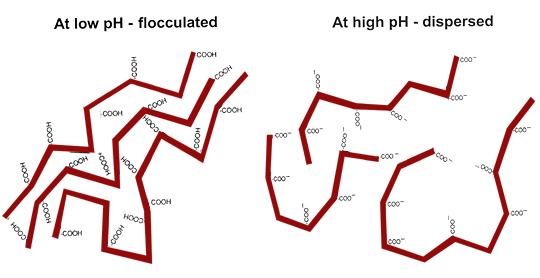
Particles with a thick DDL tend to DISPERSE.
Particles with a thin DDL tend to FLOCCULATE.
Cation Exchange
Below is an example of a cation exchange where K and Na are the only cations present in a system with clay colloids:
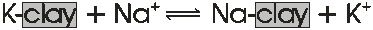
Ion exchange reactions like the one above are:
- Rapid
- Reversible
- Stoichiometric
If a few Na+ ions are added to the solution, the above equation goes to the right. As the amount of Na+ ions increases, the probability that Na+ will displace K+ also increases. If an enormous number of Na+ ions are added, then there is a high probability that nearly all of the adsorbed K+ will be replaced. This phenomenon is called the mass ion effect.
Cation Exchange Capacity
Exchangeable cations are those cations that are readily displaced by mass ion effect from negatively charged colloids on which they are adsorbed.
Cation exchange capacity (CEC) refers to the number of exchangeable cations that soil solids can adsorb. CEC is expressed as moles of positive charge adsorbed per unit mass (cmolc/kg = Centimoles of charge per kilogram of soil). An obsolete unit is meq/100g (meq/100g = Milliequivalents of charge per 100g of dry soil).
Ranges of CEC
| Colloid | Approximate CEC (cmolc/kg) |
|---|---|
| Kaolinite | 3 - 15 |
| Montmorillonite | 100 |
| Fe/Al oxides (sesquioxides) | 3 |
| Organic Matter (humus) | 150 - 250 |
| Amorphous minerals | 5 - 350 |
| RELATED LINK:
To learn more about how to determine cation exchange capacity, visit the Virtual Soil Lab Module |
Percentage Base Saturation
Ca2+, Mg2+, K+, NH4+, and Na+ are called base cations or base-forming cations. In soil, their salts undergo hydrolysis, which increases alkalinity by the release of OH- ions into soil solution. When adsorbed by soil colloids in the place of H and Al ions, base cations reduce soil acidity and increase soil pH. For example:
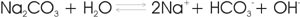
Base saturation (BS) refers to the fraction of cation exchange sites occupied by base cations and is usually expressed as a percentage. Since Ca, Mg, K, and Na account for the majority of total exchangeable bases in most soils, these are sometimes the only ones measured for estimation of base saturation.
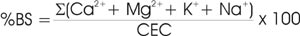
Anion Exchange Capacity
Soils in which the predominant colloids are sesquioxides (oxides of Fe and Al) may have a net positive charge. This creates opportunities for anion adsorption and exchange.
A clay-size particle of hydrous aluminium oxide has a positive charge under acid conditions, as indicated in the following reaction and therefore contributes to the soil's anion exchange capacity (AEC). Materials like sesquioxides may acquire a pH-dependent charge.
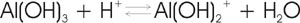
At sufficiently high pH, sesquioxides can contribute to cation exchange capacity by acquiring extra hydroxyl groups. However, most soils abundant in sesquioxides are naturally acidic and as such contribute to AEC.
| Colloid | Approximate AEC (cmolc/kg) |
|---|---|
| Kaolinite | 3 - 15 |
| Gibbsite (Al) | 5 |
| Goethite (Fe) | 5 |
| Allophane | 15 |