LFS:SoilWeb/Soil Components/Mineral Components
Mineral particles are inorganic materials derived from rocks and minerals. They are extremely variable in size and composition.
Composition
Types of Primary and Secondary Minerals
Primary minerals are formed at high temperature and pressure, under reducing conditions without free oxygen. They have not been altered chemically since deposition and crystallization from molten lava. The primary minerals are mainly present in soils as sand and silt particles.
Photos of common primary minerals present in soils are shown below:
-
Quartz
SiO2 -
Feldspar, Group: Microcline
MAlSi3O8 where M = Na, K, Ca -
Primary Mica, Group: Muscovite
KAl(AlSi3O10)(OH)2 -
Primary Mica, Group: Biotite
K(Mg, Fe)(AlSiO10)(OH)2
-
Pyroxene: MSiO3,
where M = Mg, Mn -
Olivine: (Mg, Fe)2SiO4
-
Amphibole: Tremolite
Ca2Mg5Si8O22(OH)2
Secondary minerals are formed at low temperature and pressure through oxidation. They are the weathering product of primary minerals, either through alteration of their structure or through re-precipitation. Secondary minerals are usually present in soil as clay particles.
The most abundant secondary minerals in soils are:
- Silicate clay minerals or aluminosilicates
- Soluble minerals such as carbonates and sulfates
- Oxides or hydroxides of Al and Fe (so called sesquioxides)
- Amorphous or non-crystalline minerals
Mineral vermiculite, a silicate clay mineral in the phyllosilicate sub-group:
Mineral calcite-dolomite-gypsum, soluble carbonate minerals:
Mineral goethite-hematite, hydroxides of Fe:
Mineral allophane, an amorphous mineral:
RELATED LINKS:
|
Weathering
Weathering refers to the breakdown and changes in rocks and sediments at or near the Earth's surface brought about by biological, chemical, and physical agents or combinations thereof. Weathering also involves the synthesis of new (secondary) minerals that are of great importance in soil (e.g., clay minerals).

RELATED LINKS:
|

Physical weathering is set of processes that causes the disintegration of rocks without chemical change. However, chemical and physical weathering often occur together. Physical weathering can occur due to temperature and pressure changes, freezing and thawing action, etc. For example, cracks in rocks formed by changes in temperature can be widened by freezing and thawing of water that accumulates in those cracks. Furthermore, cracks created by physical weathering will increase the surface area exposed to chemical action, thus amplifying the rate of rock disintegration.
Examples of physical weathering include processes such as crystal growth, thermal expansion, moisture swelling, abrasion, etc.
Chemical weathering changes the composition of rocks, often transforming them when water interacts with minerals to create various chemical reactions. Chemical weathering is a gradual and ongoing process that changes minerals within the rocks, through which new or secondary minerals develop from the original minerals of the rock.
Chemical weathering consists of the following processes:
1) Hydration: Intact water molecules bind to a mineral transforming hematite into ferrihydrate

2) Hydrolysis: Water molecules split into their hydrogen and hydroxyl components and hydrogen replaces a cation from the mineral structure (e.g., transformation of feldspar to kaolinite)

RELATED LINKS:
|
3) Dissolution (or solution): Water is capable of dissolving many minerals by hydrating the cations and anions until they become dissociated from each other and surrounded by water molecules. (e.g., dissolution of gypsum):

4) Carbonation: Weathering is accelerated by the presence of acids that increase the activity of hydrogen ions in water. For example, when carbon dioxide dissolves in water (a process enhanced by microbial and root respiration) the carbonic acid (H2CO3) produced hastens the chemical dissolution of calcite into limestone (or marble):
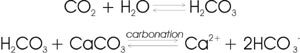
5) Oxidation-reduction: Minerals that contain Fe, Mn, or S are especially susceptible to oxidation-reduction reactions. When, for example, iron is oxidized from divalent to trivalent form, the change in valence and ionic radius causes destabilizing adjustments in the crystal structure of the mineral.

Biological weathering is due to activity of various plants and animals that release of acidic compounds. For example, lichens growing on rocks are exhibiting effects on both physical and chemical weathering processes. The physical effects are through the mechanical disruption of rocks caused by hyphal penetration, expansion and contraction of lichen thallus, swelling action of the organic and inorganic salts originating from lichen activity. Lichens also have significant impact in the chemical weathering of rocks by the excretion of various organic acids (e.g., oxalic acid), which dissolve minerals and chelate metallic cations.
Biological weathering effects include:
- The break up of rock particles by roots
- The transfer and mixing of materials by burrowing animals
- The formation of organo-mineral complexes (soil biological processes produce organic acids that can solubilize Al and Si ions, which are removed from a mineral by this process).
Most Common Elements in Soils
The elements that are found in soils in the highest quantities are O, Si, Al, Fe, C, Ca, K, Na, and Mg. These are also major elements found in the Earth's crust and in sediments. Oxygen is the most prevalent element in the Earth's crust and in soils. It comprises about 47% of the Earth's crust by weight and more than 90% by volume.
The median and range of various elements present in soils from around the world are shown in the table below.
| Element | Soils (mg/kg) | Soils (mg/kg) | Earth's Crust (mean) | Sediments (mean) |
|---|---|---|---|---|
| Median | Range | |||
| O | 490,000 | - | 474,000 | 486,000 |
| Si | 330,000 | 250,000 - 410,000 | 277,000 | 245,000 |
| Al | 71,000 | 10,000 - 300,000 | 82,000 | 72,000 |
| Fe | 40,000 | 2,000 - 550,000 | 41,000 | 41,000 |
| C (total) | 20,000 | 7,000 - 500,000 | 480 | 29,400 |
| Ca | 15,000 | 700 - 500,000 | 41,000 | 66,000 |
| Mg | 5,000 | 400 - 9,000 | 23,000 | 14,000 |
| K | 14,000 | 80 - 37,000 | 21,000 | 20,000 |
| Na | 5,000 | 150 - 25,000 | 23,000 | 5,700 |
| Mn | 1,000 | 20 - 10,000 | 950 | 770 |
| Zn | 90 | 1 - 900 | 75 | 95 |
| Mo | 1.2 | 0.1 - 40 | 1.5 | 2 |
| Ni | 50 | 2 - 750 | 80 | 52 |
| Cu | 30 | 2 - 250 | 50 | 33 |
| N | 2,000 | 200 - 5,000 | 25 | 470 |
| P | 800 | 35 - 5,300 | 1,000 | 670 |
| S (total) | 700 | 30 - 1,600 | 260 | 2,200 |
Particle and Bulk Density
Particle density (ρs) is mass of solids (Ms) per volume of solids (Vs).
| ρs= Ms/Vs |
In most mineral soils, the mean density of particles is about 2.6-2.7 g/cm3 (or 2600-2700 kg/m3). Soils with a high content of iron oxides and various heavy minerals have a particle density of 5.2-5.3 g/cm3, while soils with high organic matter content can have a particle density as low as 1.3 g/cm3.
Bulk density (ρb) is the mass of solids (Ms) per total soil volume (Vt).
| ρb = Ms / Vt
ρb= Ms / (Vs + Va + Vw) Vs = volume of solids; Va = volume of air; Vw = volume of water |
Bulk density is always smaller than ρs. Since in a general case pores constitute half the volume, ρb is about half of ρs, namely 1.3-1.35 g/cm3 (or 1300-1350 kg/m3).
Size of Soil Particles
The mineral particles present in soils vary enormously in size from boulders and stones down to sand grains and minute clay particles that cannot be seen by an optical microscope. An arbitrary division is made by size-grading soil into:
a) material that passes through a sieve with 2 mm diameter holes - the fine earth (consisting of sand, silt, and clay particles),
b) material that is retained on the sieve (> 2 mm) - the coarse fragments (gravel, cobbles, and stones).
Coarse fragments (diameter > 2 mm) are defined as rock fragments and do not include fragments of pads or concretions.
Soil Texture
Soil texture refers to the relative proportions of sand, silt, and clay in a soil. It is often the first and most important property to be determined when describing a soil, since many conclusions can be drawn from this information (e.g., water intake or infiltration, water storage in the soil, soil aeration, soil fertility, trafficability, etc.).
A number of different classification systems have been devised to divide soil particles according to their size. The figure below shows the limits of different size classes in three systems of soil particle size classification. Although the Canadian classification system is not shown, it is very similar to the USDA system, with the exception of sub-classes of silt and clay size classes.

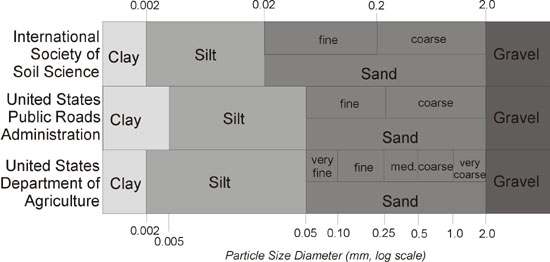
Textural names are given to soils based on the relative proportions of each of the three soil particles - sand, silt, and clay. Soils that contain predominantly clay size particles are called clay (textural class), those with high silt content are called silt (textural class), and those with a high sand content are called sand (textural class). A more complicated soil textural class that is called a loam is a mixture of sand, silt, and clay particles and it exhibits the properties of those particles in about equal proportions.
Soil textural classes can be assessed by the hand-texturing method. This method involves taking a small sample of soil, sufficient to roll into a ball of about 2.5 cm in diameter. Using a small amount of water, the sample is then moistened to the sticky point (the point at which it begins to adhere to the fingers). The ball is then molded to determine its workability and its class according to the steps in the chart entitled “Key for soil texture determination” shown at the right.
Soil Colloids
Types of Soil Colloids
When suspended in water, colloids do not readily settle out due to Brownian motion (when particles are moved around by collisions with other small particles). In chemistry, colloids are particles smaller than 0.001 mm (or 1 μm), but it has been observed that soil particles smaller than 0.002 mm (or 2 μm) still behave in the same manner, hence they are called soil colloids.
Most common soil colloids are:
- Phyllosilicate clay minerals
- Oxides and hydrous oxides of iron and aluminum (e.g., gibbsite, goethite, hematite)
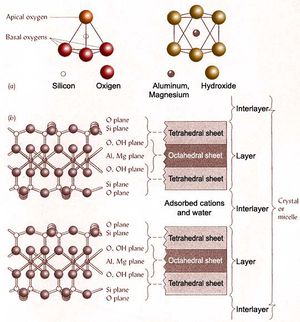
The basic molecular and structural components of silicate clays. (a) Single tetrahedron and single octahedron. (b) Thousands of tetrahedrons and octahedrons are connected to give planes of silicon and aluminum (or magnesium) ions. - Amorphous minerals (e.g., allophane and imogolite)
- Organic colloids
Phyllosilicate Clay Minerals
Phyllosilicate clay minerals are a sub-group of silicates (an extensive group of minerals which are derived from silica SiO2). All silicates are based on the (SiO4)4- tetrahedron with partial or complete sharing of oxygen atoms. Different silicate structures arise from the various ways in which (SiO4)4- tetrahedra combine with one another. Two types of building blocks are present in phyllosilicates:
- tetrahedron (4-faced structure) where each silicon (Si) atom is surrounded with 4 oxygen atoms
- octahedron (8-faced structure) where each aluminum (Al) atom is surrounded by 6 oxygen atoms
Two types of phyllosilicate clay minerals that are commonly present in soils are montmorillonite and kaolinite.

MONTMORILLONITE is a 2:1 phyllosilicate mineral where there is one Al-octahedral sheet sandwiched between two Si-tetrahedral sheets (one such sandwich is called a layer, while many layers make a micelle or crystal). The layers in montmorillonite crystals are loosely held together by very weak oxygen-to-oxygen and cation-to-oxygen bonds. Hence, water molecules and various ions are attracted between layers (into the interlayer space) causing expansion of the crystal. Montmorillonite is characterized by pronounced swelling when wet and shrinkage on drying. Wide cracks commonly form as montmorillonite-dominated soils (e.g., Vertisols) are dried. Typically, montmorillonite particles are thin (thickness ~1 to 3 nm and width ~500 nm), flat disks.
KAOLINITE is a 1:1 phyllosilicate mineral in which there is one Al-octahedral sheet bonded to one Si-tetrahedral sheet by shared (apical) oxygen atoms of the tetrahedral sheet. Two adjacent layers are held together by hydrogen bonding. Consequently, the structure is fixed and no expansion occurs between layers when the clay is wetted. The effective surface of kaolinite is restricted to its external surface area. The typical kaolinite particle is fairly large (thickness ~50 nm and width ~1-3 nm) in comparison with other clay particles and hexagonal in shape. Because of the strong binding forces between their structural layers, kaolinite particles are not readily broken down into extremely thin plates. Kaolinite exhibits very little shrinkage and swelling.

SEM image of a kaolinite crystal.

Models of the 1:1-type clay, kaolinite.
Amorphous (or Non-Crystalline) Minerals
Imogolite and allophane are minerals found in many soils including soils formed on volcanic ash parent material and the Podzolic soil type. They are very small (about 2 nanometers in diameter) and a very reactive component of soils.
Imogolite and allophane are amorphous, which means that they do not have a repetitive structure with atoms arranged in a regular pattern over relatively long distances (at least tens of nanometers). Because their structure is somewhat ordered on a few nanometers scale, they are said to be 'paracrystalline' or to have 'short range order'.
Imogolite and allophane are both aluminosilicates, which means that they are made of variable amounts of aluminum (Al), silicon (Si), oxygen (O), and hydrogen (H) ions. They have a rather similar composition but their morphology is different. Imogolite forms tubes while allophane forms hollow spheres.
Specific Surface Area & Charge Formation
Specific surface area is defined as the surface area of particles per unit mass or unit volume of particles. Because of their large size, sand particles have small specific surface area (e.g., 0.1 m2/g). Clay particles, on the other hand, have a very large specific surface area, giving them a tremendous capacity to adsorb water and other substances (e.g., 10 - 1,000 m2/g).
Soil colloids often have charged surfaces. Silicate clay minerals such as phyllosilicates end up with charges due to a process known as isomorphic substitution.
Isomorphic substitution is the process by which one ion fills a position within the crystalline structure of a mineral, that was filled by another ion of similar size and valence. This process leads to formation of the permanent charge on clay minerals.
The "ideal" montmorillonite composition is HSi2AlO6, which results in an ideal net charge of [1(+1) + 2(+4) + 1(+3) + 6(-2)] = 0. However, the real composition of montmorillonite involves isomorphic substitution of Mg for some Al in the octahedral sheet. With Mg2+ substituting for Al3+ there are fewer positive charges to neutralize the negative charges and a large permanent (negative) charge results (it is called permanent charge since it takes place during crystallization and it is not subject to changes). In contrast, there are also variable or pH-dependent charges that are formed by protonation and deprotonation of functional groups like -OH.
The "ideal" kaolinite composition is H4Si2Al2O9, which results in an ideal net charge of [4(+1) + 2(+4) + 2(+3) + 9(-2)] = 0. The composition of real kaolinite is close to ideal, since there is very little or no isomorphic substitution, resulting in no permanent charges. Kaolinite in soil usually has some surface contamination, and net negative charge is about 8 cmolc/kg soil. In addition, kaolinite has a small amount of variable charge related to dissociable OH groups attached to Al at the edges of the crystal, where the structural regularity ends.











