Course:SPPH381B/TermProject/Tylenol -Alex Kitt/Petroleum Refining
Primary Manufacturing Overview
McNeil Consumer healthcare, a division of Johnson and Johnson, synthesize acetaminophen from nitrobenzene, a benzene derivative [1]. In order to obtain this benzene, catalytic reforming of naphtha (a crude oil distillate) is most commonly used in the US [2]. Crude oil distillation and catalytic reforming, along with other processes, are performed at petroleum distillation plants.
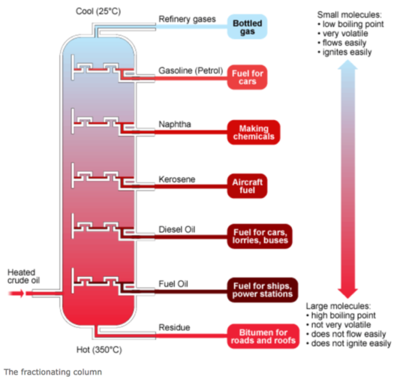
Crude Oil Distillation
Fractional distillation of crude oil separates the oil based on boiling point, and one of the fractions produced is naphtha [3]:
Catalytic Reforming
Catalytic reforming involves the addition of hydrogen and a catalyst, and then heating to between 425-530 degrees Celsius and 7-35 bar pressure [4]. This causes the cyclisation of linear hydrocarbons, such as hexane [5]. The resulting benzene can then be extracted using aromatic extraction [6].
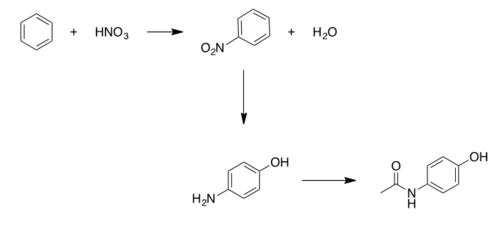
Benzene to Acetaminophen Synthesis
While there are many different reaction schemes to produce acetaminophen, the first industrialized process developed by Mallinckrodt (which is also one of the largest producers) follows a scheme with a p-aminophenol intermediate [7].
Steps:
1) Conversion of Benzene to Nitrobenzene via nitrogenation, uses concentrated sulphuric acid (catalyst) and nitric acid. [8]
2) Conversion of Nitrobenzene to para-aminophenol via hydrogenation, uses a Pt/C catalyst [9].
3) Conversion of para-aminophenol to Acetaminophen, uses acetic acid, carbon, and acetic anhydride to produce a relatively pure final product [10].
Hazards Associated with Petroleum Refining
Following extraction, crude oil must be processed in order to obtain benzene at an oil refinery plant. According to the US Bureau of Labor Statistics, only 48 people died between 2006-2015, and a further 400 people suffered non-fatal injuries and illnesses [11]. However, the US Bureau of Labor Statistics did not account for contract worker deaths until 2011, so the death toll likely underrepresents the actual number of fatalities. The causes of injuries and fatalities are caused by numerous hazards:
Physical
- Noise: caused by loud coolers, fans, and turbines
- Vibration: many of the machines (coolers, fans, and turbine) which produce loud noise also produce vibration. Additionally, hand tools may cause Hand Arm Vibration (HAV)
- Microclimatic conditions: due to reaction conditions and heating of machines, microclimatic conditions can occur
- Crushing injuries: Crushing injuries by worksite vehicles and during distiller maintenance is a hazard
- Fire and explosion: for more on fire and explosion hazards, see here.
- Struck-by: while doing maintenance and repairs around the distillery plant, workers are at risk for struck-by accidents due to falling objects, moving vehicles, and more.
Mechanical and Ergonomic
- Repetitive motion: Many daily processes involve repetitive motion, which may cause strain and other issues over time
- Heavy lifting: Much of the equipment used in maintenance and processes involved with the distillate are heavy and can cause ergonomic strain if not carried properly
Chemical
Many chemicals found and used in the petroleum refinery industry are hazardous, including: benzene, carbon monoxide, sulphur dioxide, and high boiling aromatic hydrocarbons. These chemicals may be irritants, corrosive, carcinogenic, or have other damaging effects. [12]. These chemicals vary in their hazards, some being carcinogenic, toxic, or asphyxiants.
References
- ↑ NIIR Borad of Consultants and Engineers. (n.d.) Drugs and Pharmaceutical Technology Handbook. Asia Pacific Business Press, Inc. Retrieved from https://books.google.ca/books?id=8GHDCwAAQBAJ&pg=PT44&lpg=PT44&dq=mallinckrodt+hydrogenation+of+nitrobenzene&source=bl&ots=808M-PG-u1&sig=GaMobjtuuQSjCaZyflgrL0zCYAM&hl=en&sa=X&ved=0ahUKEwiwsteEh_DSAhUIy2MKHTp4DmMQ6AEIMjAD#v=onepage&q=mallinckrodt%20hydrogenation%20of%20nitrobenzene&f=false
- ↑ ICIS. (2007, November 1). Benzene Production and Manufacturing Process. Retrieved from https://www.icis.com/resources/news/2007/11/01/9075160/benzene-production-and-manufacturing-process/
- ↑ BBC. (n.d.) Fractional distillation. Retrieved from http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/rocks/fuelsrev3.shtml
- ↑ ICIS. (2007, November 1). Benzene Production and Manufacturing Process. Retrieved from https://www.icis.com/resources/news/2007/11/01/9075160/benzene-production-and-manufacturing-process/
- ↑ Clark, J. ChemGuide.(2016) Manufacturing Arenes. Retrieved from http://www.chemguide.co.uk/organicprops/arenes/manufacture.html
- ↑ Netzer, D., Ghalayini, O. (2003). NPRA. Benzene Recovery from Refinery Sources by Co-production of Olefins. Retrieved from http://www.petrochemicals.dnetzer.net/3.23.03-benzenePres.pdf
- ↑ Caskey, J., Chapman, D. (1986). US Patents. Process for preparing p-aminophenol and alkyl substituted p-aminophenol. Retrieved from https://www.google.com/patents/US4571437
- ↑ Clark, J. ChemGuide.(2016). NITRATION OF BENZENE AND METHYLBENZENE. Retrieved from http://www.chemguide.co.uk/organicprops/arenes/nitration.html
- ↑ Caskey, J., Chapman, D. (1986). US Patents. Process for preparing p-aminophenol and alkyl substituted p-aminophenol. Retrieved from https://www.google.com/patents/US4571437
- ↑ Schulman, H., Baron, F., Weinberg, A. (1975). US Patents. Preparation of N-acetyl-p-aminophenol. Retrieved from http://www.freepatentsonline.com/3917695.html
- ↑ US Bureau of Labor Statistics. Injuries, Illnesses, and Fatalities. Retrieved from https://www.bls.gov/iif/
- ↑ Infrastructure Health & Safety Association. Oil Refineries and Petrochemical Plants. Retrieved from: https://www.ihsa.ca/rtf/health_safety_manual/pdfs/locations/Oil_Refineries.pdf