Course:MEDG550/Student Activities/Waardenburg Syndrome Type I
Waardenburg Syndrome Type I (WS1), is a genetically inherited type of syndromic hearing loss. Other features of this condition include pigmentation abnormalities and dystopia canthorum (seemingly widely spaced eyes). WS1 occurs in about 1:20,000 to 1:40,000 live births, comprising 3% of all deaf children [1].
Clinical Features

P. J. Waardenburg first described WS1 in 1951, highlighting key features of the condition that remain part of the clinical diagnostic criteria today [2][3]. In order for an individual to be diagnosed as affected, they must meet two major criteria or one major plus two minor criteria [3][1].
Major Criteria
- Congenital sensorineural hearing loss. Congenital means that the hearing loss is present from birth. Sensorineural hearing loss is a specific types of hearing loss that happens as a result of damage to the nerves in the innermost part of the ear, which are the nerves that allow our brains to process sound[4].
- Iris pigmentation abnormalities. This includes three main possibilities: 1) Two eyes of different colours, 2) Two or more different colours in one eye, or 3) Brilliant blue eyes.
- Hair hypopigmentation (loss of pigmentation). Oftentimes, this can manifest as a white lock of hair on one's head, which may appear at any age and may disappear and repear throughout one's life. Body hair, such as eyebrows and eyelashes can be white or very lightly coloured.
- Eyes that seem widely spaced. This is due to increased space between the inner corners of the eyes.
- First-degree relative previously diagnosed with WS1. First degree relatives include parents, full siblings, and children.
Minor Criteria
- Several areas of hypopigmented skin
- Eyebrows that connect in the middle
- Broad, high nasal root (space at the top of the nose and between the eyes is broad)
- Underdeveloped outer cartilage of the nostrils (hypoplasia of alae nasi )
- Premature graying of the hair, occurring before 30 years of age
Inheritance
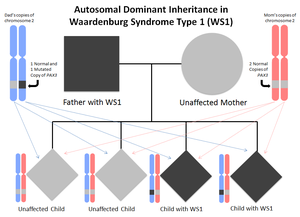
WS1 is inherited in an autosomal dominant manner. This means that to be affected with WS1, an individual needs to inherit one copy of a gene with a change on it, causing WS1. This gene with a change casuing WS1 can be inherited from the individuals biological mother or father. Typically, the parents this copy of the gene is inherited from is also affected with WS1. Occasionally, an individual may be found to have this genetic change when neither of their parents had WS1. In these cases, the genetic change is new for the first time in this individual (this is called a de novo mutation).
Every individual has 2 copies for every gene that makes up their genome. One copy was passed down from their father in their father’s sperm, and one copy was passed down from their mother via the egg. Accordingly, when we make either sperm or eggs, we pass one of each copy of our genes into the egg or sperm, and which copy the egg or sperm receives is random. Thus, for those affected, because there is a 50% chance that the egg/sperm receives either the normal or the mutated copy of the causal gene, there is a 50% chance of passing the condition on and having an affected offspring.
Genetics
WS1 is caused by mutation in the PAX3 gene (OMIM# 606597), which is located on the long arm of chromosome 2 [1]. PAX3 belongs to a group of other proteins, which play a role in embyronic development of nerves, facial strctures, and cells that produce pigement [5]. When this gene is not activated due to a genetic change, nerves, facial structures, and pigement may not be able to develop typically, as we see in WS1. Approximately 96% of individuals with WS1 have a change in their PAX3 gene. Changes in the PAX3 gene can ussually be detected on genetic tests.The remaining 4% are thought to have genetic changes that have not be been to be detected to date[6].
Differential Diagnoses
Another form of Waardenburg Syndrome, Waardenburg Syndrome Type II (WS2), has similar features and may be easily confused with WS1. WS1 can be distinguished from WS2 by the presence of eyes that appear widely spaced apart, which occurs only in WS1 [1]. White forelock, patches of white skin, and high nasal root are also more prevalent in WS1 than WS2, although sensorineural hearing loss and heterochromic iridum are more prevalent in WS2 [1]. WS2 is caused by mutations of MITF or SOX10 [1].
Waardenburg Syndrome Type III (WS3), in contrast, has dystopia canthorum and many of the other features of WS1, but also features upper limb abnormalities including contractures of the limb muscles or joints, carpal bone fusion, or syndactyly [1][7]. Similar to WS1, WS3 is also caused by mutations in PAX3 [1][8].
Waardenburg Syndrome Type IV (WS4), has the additional feature of Hirschsprung disease on top of pigmentation abnormalities and hearing loss [1][7]. WS4 is caused by mutations in EDNRB, EDN3, or SOX10 [1].
Genotype-Phenotype Correlations
To date, genotype/phenotype correlations in the PAX3 gene are not perfectly characterized. Some observations have nonetheless been made in the scientific literature:
- An Asn→His mutation at position 47 is an established pathogenic variant in WS3 [1][8].
- PAX3 pathogenic variants that delete the homeodomain (as opposed to pathogenic variants that include missense mutations or deletions in the paired domain) are associated with pigmentation disturbance [1].
- presence of eye pigment abnormalities were more strongly associated with individuals carrying deletions of the PAX3 homeodomain and the Pro-Ser-Thr-rich region compared to individuals with an amino acid substitution in the homeodomain [9].
- No genotype-phenotype correlation for the hearing loss in WS1 has been found [1].
- Size of the PAX3 gene deletion does not appear to be predictive of gene severity [1].
Mangement/Treatment
There is no cure for WS1. Early diagnosis is important so that individuals can access resources for hearing loss if needed. Some individuals with WS1 may qualify for a cochlear implant, which is a device surgically placed in the ear and allows individuals to process sound. This is the only definitive treatment for sensorineural hearing loss in WS1. It is important that individuals use sun protection, espeically for hypopigemented patches of skin, as this lighter pigmentaiton may put them at higher risk for developing skin cancer when it is not protected from the sun[10].
Genetic Counselling
Since WS1 is inherited in an autosomal dominant manner (see “Inheritance” above), this means that the majority of individuals affected by WS1 tend to have an affected parent, and that they also have a 50% chance of passing the condition on to their own offspring. Accordingly, siblings of an affected individual with an affected parent have a 50% chance of being affected as well.
In a small proportion of cases, the affected individual will not appear to have an affected parent. This has several implications, both medical and non-medical:
- For the affected individual, the causative PAX3 mutation arose spontaneously in either the sperm or egg that their mother or father provided. In other words, the parents of the affected individual are not likely to be carriers of the PAX3 mutation, and they are unlikely to have more children who are similarly affected.
- There is also a possibility of alternate paternity or maternity (if there was assisted reproduction involving egg donation, as an example).
- That the affected individual has been adopted, and for whatever reason, this has not been explicitly communicated.
If a family affected by WS1 has had genetic testing and the particular PAX3 variant that is being passed down has been identified, then prenatal testing is an option, should the parents which to determine if a fetus is affected by WS1 or not. It’s important to note, however, that knowing the PAX3 mutation status doesn’t necessarily mean the severity of the baby’s symptoms can be predicted. WS1 is extremely variable from one individual to the next, with symptoms ranging from mild to moderate. This includes hearing loss, which may or may be present in different affected individuals belonging to the same family.
Resources
For more information on Waardenburg Syndrome and other forms of hereditary hearing loss, visit:
- NCBI Genes and Disease: Waardenburg Syndrome Type I http://www.ncbi.nlm.nih.gov/books/NBK1531/
- NCBI Genes and Disease: Deafness and Hereditary Hearing Loss Overview http://www.ncbi.nlm.nih.gov/books/NBK1434/
To connect with others affected by Waardenburg Syndrome, and access other resources, visit:
- Waardenburg Syndrome Support Group: http://www.dailystrength.org/c/Waardenburg-Syndrome-WS/support-group
- Canadian Association for the Deaf: http://www.cad.ca/
- National Organization of Albinism and Hypopigmentation (NOAH): http://www.albinism.org
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 GeneReviews®, Milunsky JM. Waardenburg Syndrome Type I. 2001 Jul 30 [Updated 2014 Aug 7]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2015.
- ↑ Waardenburg PJ. A New Syndrome Combining Developmental Anomalies of the Eyelids, Eyebrows and Nose Root with Pigmentary Defects of the Iris and Head Hair and with Congenital Deafness - Dystopia-Canthi Medialis Et Punctorum Lacrimalium Lateroversa, Hyperplasia Supercilii Medialis Et Radicis Nasi, Heterochromia Iridum Totalis Sive Partialis, Albinismus Circumscriptus (Leucismus, Poliosis), Et Surditas Congenita (Surdimutitas). Am J Hum Genet 1951;3(3):195-253.
- ↑ 3.0 3.1 Farrer LA, Grundfast KM, Amos J, Arnos KS, Asher JH, Beighton P, et al. Waardenburg Syndrome (Ws) Type-i is Caused by Defects at Multiple Loci, One of which is Near Alpp on Chromosome-2 - 1st Report of the Ws Consortium. Am J Hum Genet 1992 MAY;50(5):902-913.
- ↑ Tanna RJ, Lin JW, De Jesus O. Sensorineural Hearing Loss. [Updated 2023 Aug 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK565860/
- ↑ Genetics Home Reference, National Library of Medicine (US). Genetics Home Reference [Internet]. Bethesda (MD): The Library; 2015 Mar 10. PAX3; [reviewed 2012 Aug; cited 2015 Mar 12]. Available from: http://ghr.nlm.nih.gov/gene/PAX3
- ↑ Milunsky JM. Waardenburg Syndrome Type I. 2001 Jul 30 [Updated 2022 Oct 20]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1531/
- ↑ 7.0 7.1 Online Mendelian Inheritance in Man, OMIM®, Johns Hopkins University, Baltimore, MD. MIM Number: {193500}: {04/03/2012}: . World Wide Web URL: http://www.omim.org/entry/193500
- ↑ 8.0 8.1 HOTH C, MILUNSKY A, LIPSKY N, SHEFFER R, CLARREN S, BALDWIN C. Mutations in the Paired Domain of the Human Pax3 Gene Cause Klein-Waardenburg Syndrome (Ws-Iii) as Well as Waardenburg Syndrome Type-i (Ws-I). Am J Hum Genet 1993 MAR;52(3):455-462.
- ↑ DeStefano A, Cupples L, Arnos K, Asher J, Baldwin C, Blanton S, et al. Correlation between Waardenburg syndrome phenotype and genotype in a population of individuals with identified PAX3 mutations. Hum Genet 1998 MAY;102(5):499-506.
- ↑ Ahmed jan N, Mui RK, Masood S. Waardenburg Syndrome. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560879/