Course:MEDG550/Student Activities/Spinal Muscular Atrophy
Spinal muscular atrophy (SMA) is a group of inherited disorders characterized by muscle weakness that worsens with age, resulting from the loss of motor neurons in the spinal cord and brainstem. These motor neurons control voluntary muscles, causing individuals with SMA to have impaired motor function. Severity and age of onset both vary depending on the type of SMA.[1] SMA is the second most common autosomal recessive disorder in Europeans, with an incidence of 1/6,000 to 1/10,000.[2] Approximately 1/30 to 1/50 people are a carrier of the altered gene that causes SMA.
What are the signs and symptoms of SMA?
SMA is primarily characterized by muscle weakness, low muscle tone, and impaired motor function. Additional complications of SMA include poor weight gain, sleep difficulties, pneumonia, scoliosis, and joint contractures. The presence or absence of SMA disease characteristics, and the severity of those characteristics is extremely variable from person to person. SMA is divided into five subtypes, based on age of onset and maximum function attained:
Prenatal
- Onset before birth
- Severe joint contractures
- Severe muscle weakness
- 2-6 month life span
SMA I
- Onset before six months of age
- Severe muscle weakness including facial muscles and muscles required for breathing and swallowing
- Lack of motor development
- Ability to sit without support never achieved
- Average intelligence
- Life span is most often less than two years
SMA II
- Onset between six and 12 months of age
- Muscle weakness
- Ability to sit independently when placed in sitting position
- Finger trembling
- Average intelligence in childhood and above average intelligence by adolescence
- Individuals can live into their fourth decade
SMA III
- Onset in childhood after 10 months of age
- Muscle weakness concentrated in legs
- Ability to walk short distance
- Normal life expectancy
SMA IV
- Adult onset
- Muscle weakness
- Normal life expectancy[1]
Genes and Inheritance
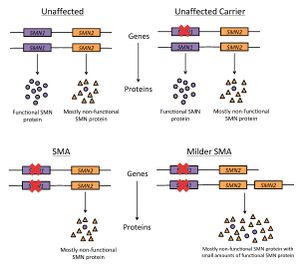

Genetic variants in the SMN1 (survival of motor neuron 1) gene can cause SMA. SMN1 is a gene that makes the SMN (survival motor neuron) protein, which is important for maintenance of motor neurons[3]. People with SMA have a mutation of both copies of their SMN1 gene which blocks production of the SMN protein. These mutations are usually inherited from parents who are unaffected carriers of SMA (they have one mutated copy of SMN1, and at least one functioning copy of SMN1)[1]. This pattern of inheritance is called autosomal recessive. Two unaffected carriers of the mutated gene have a 25% (or ¼) chance of having a child with SMA, a 25% (or ¼) chance of having a child that is unaffected and not a carrier of SMA, and a 50% (or ½) chance of having a child that is unaffected and a carrier of SMA.
SMA is also influenced by a different gene called SMN2 (survival of motor neuron 2). SMN2 is a gene that can make different versions of the SMN protein, most of which are unstable and easily broken down, and some of which are fully functional[4]. Individuals can have between zero and five copies of SMN2. People with SMA who have three or more copies of SMN2 usually have a milder subtype[1].
Diagnosis
A diagnosis of SMA is established with the following findings:
- A history of motor weakness
- Evidence of motor weakness and motor difficulties on physical examination
- SMN1 mutations identified through genetic testing
Genetic Testing:
- Most individuals (approximately 95-98%) with a diagnosis of SMA have a deletion within both copies of their SMN1 gene.
- Approximately 2-5% of individuals with SMA will have one SMN1 gene with a deletion, and one SMN1 gene with a smaller genetic change.
- Determining the number of copies of SMN2 can be used to help determine the prognosis for the individual diagnosed with SMA[1].
How are the signs and symptoms of SMA managed?
It is recommended that individual with SMA are regularly evaluated for their nutrition and feeding, respiratory function, and sleep quality. Orthopaedic evaluations are important for complications such as scoliosis and joint contractions. Quality of life can also be improved by assessing need for equipment that increases independence[1].
Are there treatments for SMA?
There is currently no cure for SMA. There are two treatment options, Spinraza and Zolgensma, that work to prevent the signs and symptoms seen with SMA. Both Spinraza and Zolgensma have been shown to improve the quality of life for people with SMA.[1]
Spinraza[1][5]
Spinraza can be used to treat anyone with SMA. Spinraza is a drug that is injected near your spinal cord and works by increasing the amount of SMN protein in your body. The increase in SMN protein has been shown to maintain or improve muscle function in clinical trials. It is important to note that treatment is lifelong with multiple injections needed every year.
Zolgensma[1][6]
Zolgensma is a one-time treatment that can be used to treat children that are less than 2 years old with SMA type 1 (SMA I). Zolgensma is a gene therapy where a working SMN1 gene is delivered to your child's cells through IV (intravenous) fluid. Having this gene therapy replace the faulty SMN1 gene with a working SMN1 gene has been shown to improve muscle function and survival.
Spinraza and Zolgensma are expensive treatments. Coverage for these treatments will vary based on where you live.
The government of Alberta has recently announced that Zolgensma will be funded by the province for children with SMA on a case-by-case basis.[7] If you live in Alberta and your child has SMA, your doctor will have more information about this funding.
Genetic Counselling
Families with a diagnosis of SMA can be referred for genetic counselling to learn more about the medical, psychological, and familial implications of the disease. Genetic counselling can educate and assist parents with genetic testing and provide support and guidance throughout the process.
Carrier Testing
If a couple with a child with SMA wishes to know the chance of having another child with SMA, genetic testing of the parents to determine if they are carriers of a SMN1 mutation can be performed.
Approximately 98% of parents with a child with SMA will be a carrier of a SMN1 mutation[1]. Two carrier parents have a 25% (or ¼) chance of having a child with SMA, a 25% (or ¼) chance of having a child that is unaffected and not a carrier of SMA, and a 50% (or ½) chance of having a child that is unaffected and a carrier of SMA (Figure 2).
For parents of a child with SMA with two SMN1 deletions, an important consideration before undergoing SMN1 deletion carrier testing is that approximately 4% of SMA carriers will have two copies of SMN1 on one chromosome, and a deletion of SMN1 on the other chromosome. Since SMN1 deletion testing is a dosage test that counts the number of SMN1 copies an individual has, these deletion carriers may be falsely identified as non-carriers. Such circumstances require further testing of additional family members before concluding that the parent is indeed a non-carrier.
Approximately 2% of parents with a child with SMA are not a carrier of a SMN1 mutation, due to a new gene mutation that occurred in the child. However, when a child with SMA appears to have a new mutation that was not inherited, there may still remain a chance that the mutation was inherited. This is due to the possibility of the mutation being present in the parent’s ovaries or testes. This phenomenon is referred to as gonadal mosaicism[1].
Prenatal Testing
Prenatal diagnosis is an option for couples with a 25% chance of having a child with SMA. A genetic test that looks for known parental SMN1 mutations in the fetus can be performed using prenatal diagnostic procedures. These include chorionic villus sampling (CVS), which is done between 10 and 12 weeks, and amniocentesis, which is done after approximately 15 weeks. Preimplantation genetic diagnosis (PGD) is also an option for such couples.
Psychosocial Concerns
SMA is complex genetic condition that, due to it heritable nature, impacts reproductive planning and decision making for known. As described above, there are many possible disease characteristics of SMA and the severity those characteristics is challenging to predict based on genetic testing. Each individual, couple, or family, will come to a genetic counselling appointment with a different past experience with SMA and different psychosocial concerns surrounding SMA.
Although each person's experience with SMA will be different, some psychosocial concerns commonly arise and range from obtaining a diagnosis, to facing premature death, to facing stigmatization or social discomfort.[1][8][9]
Diagnostic Odyssey
The differential diagnosis for SMA is large, meaning that even once doctors have noticed some of the disease characteristics of SMA there are many other conditions that need to be investigated before a diagnosis can be made. Without a diagnosis, patients or parents of patients face uncertainity with what treatments might be appropriate or how their condition might progress. On top of caring for their child with an undiagnosed condition, many parents take the lead on finding a diagnosis as many pediatricians have not seen SMA before and do not have the expertise to make a diagnosis.
Without expert input on their child's condition, or knowledge of SMA themselves, parents have the added stress and burden of trying to differentiate between characteristics of SMA and "normal" development.[9]
Family Planning
The large majority of people with SMA inherited their genetic variants from their parents. This means that for most parents who have a child with SMA, there is a 25% chance that any future pregnancies will also have SMA. Therefore, in addition to adjusting to their child's diagnosis, and coping with the fear and anxiety that can accompany that diagnosis, parents also have to cope with the implications for future children.
Immediate family members (parents or siblings) of the parents also have a 50% chance of carrying an SMA variant, and informing these family members their genetic testing can be an additional burden.[8][9]
Social Stigma
Many children and adults with SMA require a wheelchair to assist with their mobility, and face the public misconception that because they are physcially disabled they are intellectually disabled as well. Those individuals with SMA who do not use a wheelchair often face the opposite public assumption that because they do not appear "sick" they have full motor capabilities and expected to complete tasks beyond their psysical abilities. This can lead to embarrassment, discomfort, and frustration for people with SMA. People with SMA often struggle to gain the same type of government support as someone with a physical disability because of the invisible nature of their illness.[9]
Living with SMA
Many individuals with SMA are limited in participating in social activities outside of their home due different apects of their condition. This may be due to reliance on breathing equipment, concerns for lung infections (such as a sickness going around at school), or becuase the organizational burden of making sure all the necessary supports will be in place is overwhelming for parents.
Parents often feel a large amount of stress and exhaustion in caring for their children. Parents often become the primary carer for their children and receive little financial support. For example, individuals with SMA that are unable to roll over in bed, this has to be done for them by a parent or other carer to prevent bed sores. This leads to disrupted sleep for both the person with SMA and their carer.[9]
Bereavement
For parents of children of SMA Type I, there is need for ongoing psychocial support from diagnosis until the early death of their child. Many parents have feelings of guilt, helplessness, and anticipatory grief knowing the general life expectancy for their child. Parents will grieve the loss of their child in their own way. Some parents of children with SMA Type I have reported that support groups have been a helpful part of their coping. Parents have also indicated that empathy from health care providers, respecful handling of their child's body, and offers of ongoing support were appreciated during their bereavement.[8][9]
Patient and Family Resources
- Muscular Dystrophy Canada - http://muscle.ca
- Cure SMA - http://www.curesma.org
- Families of SMA Canada - http://www.curesma.ca
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Prior, T. W. & Russman, B. S. (2013). Spinal Muscular Atrophy in GeneReviews. Retrieved from: http://www.ncbi.nlm.nih.gov/books/NBK1352/
- ↑ Feldkotter, M., Schwarzer, V., Wirth, R., Wienker, T. & Wirth, B. (2002). Quantitative analyses of SMN1 and SMN2 based on real-time LightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am. J. Hum. Genet. 70, 2, 358-368.
- ↑ National Library of Medicine (US). Genetics Home Reference [Internet]. Bethesda (MD): The Library; 2015 Mar 2. SMN1; [Reviewed 2012 Aug; cited 2015 Mar 7]. Available from http://ghr.nlm.nih.gov/gene/SMN1
- ↑ National Library of Medicine (US). Genetics Home Reference [Internet]. Bethesda (MD): The Library; 2015 Mar 2. SMN2; [Reviewed 2009 Dec; cited 2015 Mar 7]. Available from http://ghr.nlm.nih.gov/gene/SMN2
- ↑ Claborn, M. K., Stevens, D. L., Walker, C. K., & Gildon, B. L. (2019). Nusinersen: A treatment for spinal muscular atrophy. Los Angeles, CA: SAGE Publications. doi:10.1177/1060028018789956
- ↑ Stevens, D., Claborn, M. K., Gildon, B. L., Kessler, T. L., & Walker, C. (2020). Onasemnogene abeparvovec-xioi: Gene therapy for spinal muscular atrophy. Los Angeles, CA: SAGE Publications. doi:10.1177/1060028020914274
- ↑ Canada, Cure SMA. "Families of Alberta children suffering from spinal muscular atrophy (SMA) may now be eligible to receive funding for gene replacement therapy treatment". Retrieved January 31, 2021.
- ↑ 8.0 8.1 8.2 Higgs, E. J., McClaren, B. J., Sahhar, M. A., Ryan, M. M. & Forbes, R. 'A short time but a lovely little short time“: Bereaved parents” experiences of having a child with spinal muscular atrophy type 1. J Paediatr Child Health 52, 40–46 (2016).
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Qian, Y. et al. Understanding the experiences and needs of individuals with Spinal Muscular Atrophy and their parents: a qualitative study. BMC Neurol 15, 217 (2015).