Course:MEDG550/Student Activities/Polycystic Kidney Disease
Polycystic kidney disease (PKD) is comprised of a group of inherited genetic conditions which lead to cyst formation in the kidneys; 1/1000 individuals are thought to have polycystic kidney disease [1]. PKD can be inherited in a number of ways, such as through autosomal recessive and autosomal dominant inheritance. Over 20 genes have been found to cause PKD [2].
Medical Description of PKD
Autosomal dominant PKD (ADPKD) is seen in 1/400 people and is the most common contributor to genetic kidney disease; ADPKD is also one of the most common causes of end-stage renal disease. [1] End-stage renal disease, or complete loss of renal function, tends to onset anywhere between the fourth and sixth decades of life [1]. Symptoms of individuals with ADPKD are highly variable, but the hallmark symptom of ADPKD is a progressive enlargement of the kidneys. The kidneys enlarge at a rate of about 5.3% per year, typically starting at birth, due to fluid build-up in multiple cysts[1]. Cysts are non-cancerous, fluid filled sacs, and in PKD they are formed in the tubular epithelium (lining) of the nephrons (basic structural unit of kidneys)[1]. Although there is progressive enlargement of the kidneys, the organ manages to maintain its function for several decades through a compensatory increase in the filtration rate in the unaffected nephrons[1]. Cyst development may also occur in other body systems, including the liver, pancreas, or seminal vesicles [2]. Common complications of ADPKD are high blood pressure, blood in your urine, frequent urination, headaches and kidney stones.
Autosomal recessive polycystic kidney disease (ARPKD) is less common that ADPKD, affecting between 1/10,000 to 1/40,000 people.[3] ARPKD is generally more severe than ADPKD. ARPKD primarily affects the liver and the kidneys. ARPKD causes congenital hepatic fibrosis, which causes malformation and scarring of the blood vessels and bile ducts of the liver, and can cause bleeding in the stomach and intestines [3]. ARPKD causes enlarged, cystic kidneys with impaired function - this can be seen with a physical exam and may be detected prenatally with ultrasound. [3] Half of patients with ARPKD will develop end-stage renal disease in their lifetime.[4] Although ARPKD is often lethal, survival of children with ARPKD is dramatically improving with the advent of regular dialysis and successful kidney transplants. Currently, 70-77% of children with ARPKD survive the first year of life,[3] and of those that survive the first year of life, there is a 82% ten-year survival rate.[5]
-
Image of cystic kidneys
Diagnosis
Autosomal Dominant PKD
Diagnosis for ADPKD is made based on several criteria. Renal imaging using ultrasound, CT or MRI will show multiple cysts and no other manifestations suggesting a different type of renal disease. Family history (suggestive of autosomal dominant inheritance) may also point to this condition. Confirmatory genetic testing may be done. On a prenatal ultrasound, a baby with ADPKD may present with enlarged echogenic kidneys, with or without cysts. These enlarged, echogenic kidneys are suggestive, although not diagnostic of ADPKD. [6]
Autosomal Recessive PKD
Diagnosis of ARPKD is made on a clinical basis. The diagnostic criteria for ARPKD are characteristic ultrasound findings (increased kidney size, abnormal brightness of the kidneys, and impaired blood flow through the medulla of the kidneys) coupled with at least one of the following: congenital heapatic fibrosis, abnormal bile ducts, or a diagnosis of ARPKD in a sibling.[7]
Management
Autosomal Dominant PKD
There is no cure for ADPKD, but there are several ways to manage the condition. Pain medications help manage the pain caused by the cysts to the back and sides of the abdomen. In severe cases, surgical options such as decompression to remove fluid may offer some relief. Individuals with ADPKD experience frequent urinary tract infections, which are treated with antibiotics. Early treatment is particularly important as it becomes much more difficult to treat once the infection reaches the cysts. Those with ADPKD are encouraged to avoid smoking, caffeine and long-term use of drugs known to be toxic to the kidneys (ex. Non-steroidal anti-inflammatory drug (NSAIDs)). Individuals with severe polycystic liver disease are also encouraged to avoid the use of estrogens.
Surveillance via imaging and other studies (ex. urine) are important to evaluate disease progression. Effective management of blood pressure has been shown to slow the progression of the condition, which may be achieved both by lifestyle changes and medication. When end-stage renal disease is reached, dialysis and transplantation are both options. Transplanted kidneys are not at risk of developing cysts in individuals with ADPKD.
Autosomal Recessive PKD
Management of ARPKD is lifelong and involves regular ultrasounds, physical exams, blood work and urinalysis to asses lung, kidney and liver function.[3] Liver enzymes, electrolyte balance, and blood pressure must be closely monitored. Most patients will require ongoing dialysis to maintain kidney function, and like with ADPKD, if end-stage renal disease develops, a kidney transplant may be required.[3]
Genetics of PKD
Autosomal Dominant PKD
There are many types of genetic disorders that cause cysts to form in the renal system, although autosomal dominant PKD (ADPKD) is the most common. ADPKD is most often caused by mutations in either the PKD1 or PKD2 genes and has an autosomal dominant inheritance pattern [2]. The PKD1 and PKD2 genes code for proteins called the polycystins, which are important regulators of the cilia in the renal system [8] Both genes show high penetrance, meaning almost everyone with a mutation in PKD1 or PKD2 will develop multiple bilateral cysts. Mutations in PKD1 account for 85% of all ADPKD cases [2]. Although rare, it is also possible that an individual will have a gene mutation in both PKD1 and PKD2, and they would be expected to have more severe kidney disease than if there was only one mutation.[3]
Autosomal dominant inheritance means that an individual only needs to inherit one mutated copy of PKD1 or PKD2 in order for them to develop PKD. Each child of an individual affected with PKD is at a 50% risk of inheriting this mutation and of developing the condition. If the child is then tested and found not to carry the gene mutation, their own children will not be at risk of developing ADPKD. The siblings of someone with a mutated gene similarly have a 50% risk of also carrying the gene. Although most cases are familial, with 95% of affected individuals having an affected parent, up to 5% of mutations detected in affected individuals appear to be de novo, or sporadic [2].
Other genes that have been found to cause ADPKD include GANAB and DNAJB11. Mutations in the GANAB gene account for ~0.3% of ADPKD, and have been associated with more mild kidney cysts, and less of a decline in kidney function. However, individuals with mutations in GANAB can present with more severe cystic liver disease.[9] Mutations in the DNAJB11 gene account for ~0.1% of ADPKD, and have been associated with smaller cysts and no kidney enlargement. These individuals may still reach end-stage kidney disease.[3]
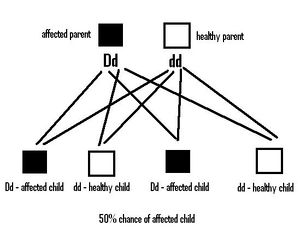
Autosomal Recessive PKD
ARPKD is caused by mutations in the PKHD1 gene. Everyone has two copies of PKHD1 - one that they inherited from their mother and one from their father. An autosomal recessive inheritance patterns means that you have to have a mutation in both copies of your PKHD1 gene to develop ARPKD, meaning that both your mother and your father have to be carriers (they each have to have one mutated copy of PKHD1 and one normal copy of PKHD1). Carriers are not expected to show symptoms or develop ARPKD because they still have one "working copy" of the PKHD1 gene.
For parents that are both carriers, there is a a 25% (1 in 4) chance of both of them passing down their mutated copies to their child, and therefore having a child that will be affected with ARPKD. There will be a 50% (2/4 or 1/2) chance that the child will similarly be a carrier with one mutated copy of PKHD1. There is a 25% (1 in 4) chance that the child will inherit both "working" or "normal" copies of PKHD1 and therefore be unaffected. In the general population 1 in 70 persons is a carrier of PKHD1.
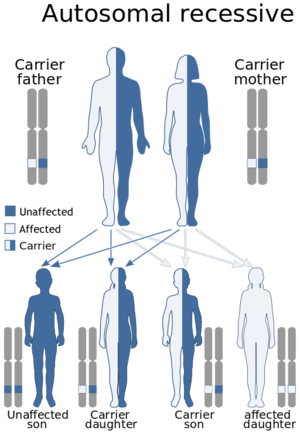
Genetic Testing
Genetic testing is able to detect 90% of PKD1 and PKD2 mutations [2]. This means that 10% of individuals with PKD will not have an identifiable mutation. There is a high degree of allelic heterogeneity underlying ADPKD, meaning that there can be many different mutations in the same gene which all cause the same clinical features. There have been over 300 different causal mutations detected in PKD1 and over 90 causal mutations in PKD2. [6] The clinical severity of this disease does not seem to depend on the type of mutation present, but rather on whether the mutated gene is PKD1 or PKD2 [2]. Mutations in PKD1 are associated with a more severe clinical phenotype due to increased cyst formation at an earlier age, with significantly earlier onset of end-stage renal disease (approximately 20 years earlier)[2]. However, there is still considerable variability in the symptoms and age of onset of ADPKD, even within the same family. This is due to the effects of the environment, different modifier genes, and the exact location of the mutation on PKD1 or PKD2 [6]. Genetic testing for GANAB and DNAJB11 are also available, but are less commonly tested for due to the small proportion of ADPKD attributed to these genes.
Genetic testing is also available for the PKHD1 gene in ARPKD, however not all mutations are detectable by genetic testing, so someone with ARPKD may still receive a negative genetic test result.[3]
Genetic Counselling Issues
Genetic testing is available to families with suspected familial PKD. Typically, testing is first offered to the affected individual in the family in order to elucidate their exact mutation and to confirm their diagnosis [6]. Following mutation identification, at-risk family members can be offered pre-symptomatic genetic testing if ultrasound imaging cannot rule out the presence of renal cysts. Prenatal testing may be offered to individuals who carry a known mutation for PKD [6].
In 10% of families, the disease-causing mutation cannot be identified. In these cases, seemingly at-risk individuals will be monitored with renal ultrasound, MRI, and/or CT. In these families, there is the possibility of mosaicism in the parent of an individual with ADPKD and these findings would have implications for the risk to siblings with an apparent de novo mutation. [6].
Carrier testing for PKHD1 may also offer information that is helpful for family planning, or identifying other family members that may be at risk of having a child with ARPKD.
Prenatal Diagnosis
For pregnancies at increased risk of PKD, molecular genetic testing is possible by amniocentesis or chorionic villus sampling (CVS) to analyze DNA from placental tissue. The disease-causing allele of an affected family member must be identified or linkage established in the family before prenatal testing can be performed [6].
Support
Living with ADPKD or having a family member with ADPKD may be extremely challenging. Support groups may provide further information on the condition and tips for management. They are an excellent source for support, allowing families to interact locally and globally.
UK
USA
Canada
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Helal I, Reed B, Schrier R. Emergent early markers of renal progression in autosomal-dominant polycystic kidney disease patients: implications for prevention and treatment. American journal of nephrology. 2012;36(2):162-7.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Harris P, Torres V. Polycystic kidney disease. Annual review of medicine. 2009;60:321-37.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 [Sweeney WE, Avner ED. Polycystic Kidney Disease, Autosomal Recessive. 2001 Jul 19 [Updated 2014 Mar 6]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2015. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1326/].
- ↑ [Gunay-Aygun, M., Avner, E. D., Bacallao, R. L., Choyke, P. L., Flynn, J. T., Germino, G. G., ... & Gahl, W. A. (2006). Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis: summary statement of a first National Institutes of Health/Office of Rare Diseases conference. The Journal of pediatrics, 149(2), 159-164.]
- ↑ [Bergmann, C., Senderek, J., Windelen, E., Küpper, F., Middeldorf, I., Schneider, F., ... & Zerres, K. (2005). Clinical consequences of PKHD1 mutations in 164 patients with autosomal-recessive polycystic kidney disease (ARPKD). Kidney international, 67(3), 829-848.]
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 [Harris PC TV. Polycystic Kidney Disease, Autosomal Dominant. Pagon RA BT, Dolan CR, et al., editors. , editor. Seattle (WA): University of Washington, Seattle; 2002 Jan 10 [Updated 2011 Dec 8].
- ↑ [Zerres, K., Rudnik‐Schöneborn, S., Deget, F., Holtkamp, U., Brodehl, J., Geisert, J., & Schärer, K. (1996). Autosomal recessive polycystic kidney disease in 115 children: clinical presentation, course and influence of gender. Acta Paediatrica, 85(4), 437-445.]
- ↑ [Boucher C SR. Autosomal dominant polycystic kidney disease. (ADPKD, MIM 173900, PKD1 and PKD2 genes, protein products known as polycystin-1 and polycystin-2). Eur J Hum Genet. 2004;12(5):347-54].
- ↑ Porath B, Gainullin VG, Cornec-Le Gall E, et al. Mutations in GANAB, Encoding the Glucosidase IIα Subunit, Cause Autosomal-Dominant Polycystic Kidney and Liver Disease. Am J Hum Genet. 2016;98(6):1193–1207. doi:10.1016/j.ajhg.2016.05.004
