Course:MEDG550/Student Activities/Osteogenesis Imperfecta
Osteogenesis imperfecta (OI), also known as brittle bone disease, refers to a group of genetic disorders characterized by bones that fracture easily. The term 'OI' means imperfect bone formation. The condition ranges from mild to severe, with some people having only a few fractures over their lifetime, while others have fractures occurring even before birth. [1]
Clinical Characteristics
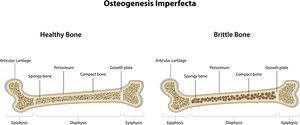
The major feature of osteogenesis imperfecta is a fragile skeleton, resulting in broken bones throughout life. Respiratory problems including asthma are often seen. Other medical problems include: bone deformity, bone pain, short height, spine curves (scoliosis), loose joints, muscle weakness, large head, hearing loss in adulthood, weak and discoloured teeth (dentinogenesis imperfecta), vision problems, easy bruising, heart problems, tiredness, and a problem in the brain known as basilar invagination. [2]
Classifications
Research has identified 16 different types of OI. [3] A person's classification may serve as an important starting point for understanding their health care needs. However, there is considerable variability among people and many do not fit perfectly into a specific type. Care must therefore be individualized to each person's specific symptoms. The most common OI classifications may be described as follows:
Type I

Type I is the mildest form of OI. It is characterized by normal height and a blue or grey tint to the eye that is normally white (the sclera). [2] Bone fractures may first occur during diapering at birth or when the infant begins to walk. There may be a few to several more fractures per year until puberty, where fewer fractures occur. The frequency of fractures may increase again during adulthood, especially in men and women over 50 years of age. [4] The total number of fractures for affected individuals may range from a few to over 100. The fractures usually heal normally so there is no resulting deformity. [2] Dislocations and sprains are common. People with type I OI may have few obvious signs of the disorder. Muscle weakness, loose joints, hearing loss, flat feet, and a disorder of tooth development known as dentinogenesis imperfecta may occur. [2] Life expectancy appears to be average.

Type II
Type II is the most severe form of OI and is also known as perinatal lethal. Infants are born with multiple fractures, an unusually soft skull and an unstable neck. [2] Infants may be small in both weight and height, and legs may fall into a frog-like position. The majority of infants with type II OI die at or shortly after birth, and survival beyond one year is rare. Death usually results from respiratory problems related to the small chest (thorax) and rib fractures. [2]

Type III
Type III OI is also known as progressively deforming, because an infant presents with mild symptoms at birth and develops worsening symptoms throughout life. [2] Fractures may occur before birth. Some infants have severe respiratory problems as a result of fractures leading to death within the first few weeks or months of life. Affected individuals who survive past infancy generally have a near-average life span. Throughout life an affected individual may have up to 200 fractures. Common features include short height, bone deformities, and a curve in the spine (scoliosis). Wheelchair use and surgical correction for these features are common. Rib cage and spine deformities may also result in respiratory problems in adulthood. [2]
Type IV
Type IV OI is the most variable form, ranging from mild to moderate. People with this form of OI may be somewhat shorter than their family members, have frequent fractures that decrease after puberty, and have mild to moderate bone deformity. [2] Hearing loss may also occur in adulthood. Life expectancy is average. [2]
Type V
Type V OI is moderate in severity and appearance is similar to type IV. Characteristic features include calluses that form at fracture sites and limited forearm rotation. [2]
Frequency
Approximately 6 to 7 in every 100,000 people have osteogenesis imperfecta. The most common forms are type I and type IV, making up more than half of all people with OI. [1] Males and females are equally affected. OI is found in all racial and ethnic groups. [2]
Genetics
All of the genetic information in our bodies can be thought of like an instruction book that provides a code for how our bodies grow, develop, and function. The genetic information is stored in smaller units of information known as genes, like pages in our instruction book. We have two copies of every gene, one from our mother and one from our father. Sometimes we have changes in our genes, like spelling mistakes on the pages of our instruction book, that effect the way the gene is able to function.
In osteogenesis imperfecta, approximately 90% of cases are caused by changes in the genes COL1A1 and COL1A2. [3] These genes provide instructions for making part of type I collagen. Collagens are proteins that provide strength and support for cartilage, bone, tendon, skin, and the white part of the eye (sclera). [5] Changes in COL1A1 and COL1A2 affect the structure of type 1 collagen, causing a weakening of bone and characteristic fractures. [1] OI types I, II, III, and IV, are all caused by changes in these two genes.
In rare cases changes in other genes are also responsible for the development of OI. These include BMP1, CRTAP, LEPRE1, P3H1, PPIB, TMEM38B, SERPINH1, FKKBP10, PLOD2, IFITM5, SERPINF1, SP7, WNT1, and CREB3L1. [3] These genes provide instructions for the processing of collagen, modification of collagen, collagen folding, bone mineralization, and bone cell development. Changes in these genes weaken bone, leading to the characteristic bone abnormalities and problems with growth. [1] OI type V through XVI are caused by changes in these genes.
For some people with osteogenesis imperfecta, there is no change found in any of the genes listed above. These cases have an unknown cause. Researchers are working to identify more genes that may play a role in the development of OI.
Inheritance
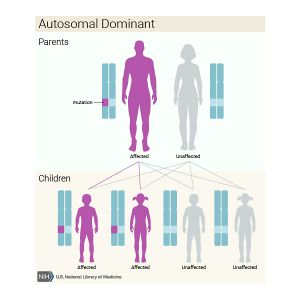
Most cases of OI are inherited in an autosomal dominant pattern. [1] This means that of the two copies of every gene that we have, only one gene with the change is necessary to cause the condition. The gene with the change is considered dominant over the normal gene. OI type I through V are all inherited in this manner. [3] Many people with mild forms of the condition, such as type I or type IV, inherit the gene change from a parent who has the disorder. For people with more severe forms of OI, such as type II and type III, there may be no family history of the disorder. This is known as de novo and occurs as a new gene change in the individual.
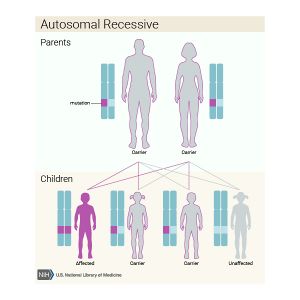
Less commonly OI is inherited in an autosomal recessive pattern. [1] This means that both genes need to have the change in order for the child to develop the condition. In this situation, the parents of the affected individual do not have OI, but they each carry one copy of the gene change. In order for a child to inherit OI in a recessive pattern, the gene change must come from both parents. OI type VI through XVI are all inherited in this manner. [3]
Diagnosis
OI is often diagnosed clinically by a physician. The presence of fractures from mild trauma and other features such as growth deficiency or blue sclera may be sufficient for diagnosis. [3] Babies with more severe forms of OI may be born with broken bones and are diagnosed at birth. More mild forms of OI may not be diagnosed until the teenage or adult years.
Genetic Testing
Family history and genetic testing can be used to confirm a diagnosis. In families with only one person affected with OI, sequencing the COL1 genes is first recommended. If no disease-causing gene change is found, this can be followed up by sequencing the other OI genes. In families with multiple affected individuals, it is recommended to investigate the whole OI gene panel rather than waiting to rule out the COL1 genes first. [3]
Genetic testing may provide a positive result, a negative result, or an uncertain result. A positive result means that a disease-causing gene change has been found. Identifying a gene change may be useful for determining survival, recurrence, heritability, and variable response to drugs for the affected individual. Other family members may also be tested to see if they are at risk. A negative result means that no disease-causing mutation has been found. This does not necessarily rule out OI, as researchers do not know all of the genes that play a role in the development of the condition. In this case, family members cannot be offered testing. Affected individuals may have further testing in the future once more information is known. An uncertain result means that a gene change has been found, but scientists do not know if the change has any affect on the function of the gene. Similarly, family members are not offered testing and affected individuals may have further testing in the future once more information is known. [6]
Prenatal Testing
Prenatal testing may be possible based on ultrasound findings or genetic testing. Ultrasound can detect fractures, shortening, and other bone abnormalities. [7] Severe forms of OI may be detected with ultrasound prior to 20 weeks gestation, while milder forms may be detected later in pregnancy when fractures or deformity occur. [7] Distinguishing between specific types of OI may not be possible using only ultrasound. Genetic diagnosis may be possible during pregnancy if the disease-causing gene change of an affected family member has been identified. [7] This may be done through chorionic villus sampling from 11 to 13 weeks gestation, or by amniocentesis after 15 weeks gestation.
Differential Diagnosis
Several conditions may present similarly to OI. Blood and urine tests may be used to rule out other bone disorders such as hypophosphatasia and rickets. Child abuse is another important differential diagnosis, as it may present with multiple fractures in various stages of healing. [8] Physical abuse occurs more frequently than OI, and on rare occasions a child may present with both abuse and OI. [9]
Treatment and Management
Currently there is no cure for OI. Treatment is based on strengthening bone, preventing fractures, enhancing independent function, and promoting general health. [10] A multidisciplinary team including the primary care doctor, physical therapist, geneticist, endocrinologist, dentist, neurologist, and orthopaedic surgeon are crucial for therapy once a diagnosis of OI is made. [10] Treatment may include fracture care, physical therapy, surgery, medication, and lifestyle management.
Initial fracture care takes a conservative approach using lightweight casting, splinting, and bracing broken bones. Long periods of immobility can further weaken bones and lead to impaired muscle function and more fractures. [10] Short term periods of immobilization are therefore preferred to allow some movement as soon as possible after the fracture. Surgery is used to promote weight bearing. It may be needed for repairing broken bones, correcting bone deformities, or repairing bones of the ear to improve hearing. [10] The most common procedure is known as rodding, where metal rods are inserted into long bones to control fractures and improve deformities. [10] Physiotherapy is critical for maximizing an individual's independent function. Typical programs include muscle strengthening and aerobic conditioning. Occupational therapy can also help with fine motor skills and selecting mobility devices such as walkers, canes, and wheelchairs. [2] Medication almost always includes bisphosphonates, which are a class of drugs used to prevent loss of bone density. [3] This drug was developed for osteoporosis, so is used off-label and parental consent is required for children. [10] Research is continuing to search for OI-specific treatment. Other medications under investigation include teriparatide and growth hormone. [3] A healthy lifestyle is important for everyone, including people with OI. This includes safe exercise and a nutritious diet. Swimming is one example of a safe form of exercise that may be beneficial for maintaining bone and muscle mass. Adequate intake of nutrients, such as vitamin D and calcium, are also important for bone health. [11] Other treatments may include hearing aids, crowns for brittle teeth, and supplemental oxygen for people with breathing problems.
Genetic Counselling
Genetic counsellors are trained professionals who provide information on genetic conditions to help people make informed personal and health decisions. Speaking with a genetic counsellor can be an important step after receiving a diagnosis of OI. Click here to find one near you.
Risk to Family Members
As described above, OI may be inherited in an autosomal dominant or autosomal recessive manner, depending on the classification.
For autosomal dominant OI (type I through V), most mildly affected individuals have an affected parent, whereas most severely affected individuals have unaffected parents. If a child with OI has an affected parent, siblings of the child have a 50% chance of also being affected with OI. If a child with OI has unaffected parents, siblings of the child have a 5% chance of also being affected with OI. [2] Individuals with type I through type V OI have a 50% chance of having a child with OI. The risk to other family members depends on if a parent is affected.
For autosomal recessive OI (type VI through XVI), parents of an affected individual are unaffected. Parents are known as carriers because they carry one copy of the gene with the change, but do not have the disorder. Siblings of an individual with OI have a 25% chance of also being affected with OI, a 50% chance of being unaffected carriers like their parents, and a 25% chance of being unaffected and non-carriers. Children of individuals with type VI through XVI OI will all be carriers.
Family Planning
A discussion of genetic risk and prenatal testing is appropriate before pregnancy. Prenatal testing may be used to help families prepare for their child or to make decisions about continuing a pregnancy. Preimplantation genetic diagnosis may also be an option for families who have an identified genetic diagnosis. This allows for selection of embryos that do not carry the change in order to have children unaffected by OI. Other options to consider include adoption, surrogacy, and egg/sperm donation. [2]
Psychosocial Issues
There are several psychosocial concerns individuals and families may face living with OI. Many people learn to cope and lead happy and fulfilling lives. Awareness of potential concerns can be helpful for preparation and implementation of support systems. Everyone is different and this list does not cover all of the concerns that people may face.
- Individuals with OI [12] [13]
- OI can impact self concept, relationships, family dynamics, and career choices
- Feelings of isolation, discrimination, anxiety, or depression
- Frustration with a lack of resources or support networks
- Frustration with inaccessibility of school, work, or social environments
- Parents of children with OI [12] [13]
- With a new diagnosis, feelings of guilt, depression, shock, relief, or uncertainty are common
- Families with a previous child with OI may have already developed coping strategies
- May be overprotective of affected children
- Frustration with health care professionals who do not handle their child correctly
- Concerns over unexplained fractures and allegations of child abuse
- Siblings of individuals with OI [12] [13]
- Jealousy and neglect over the time parents spend with the child with OI
- Worry over their sibling's hospitalizations
- Coping strategies may include: [12] [13]
- An effective support network
- Open communication among family members
- Self-care
Patient Resources
References
<references>
- National Library of Medicine (US). Genetics Home Reference [Internet]. Osteogenesis imperfecta; [reviewed April 2013; cited January 2018]. Available from: https://ghr.nlm.nih.gov/condition/osteogenesis-imperfecta
- Forlino A, & Marini JC. (2016). Osteogenesis imperfecta. The Lancet, 387(10028), 1657-1671
- Steiner RD, Adsit J, Basel D. COL1A1/2-Related Osteogenesis Imperfecta. 2005 Jan 28 [Updated 2013 Feb 14]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1295/
- Paterson CR, McAllion S, Stellman JL. Osteogenesis imperfecta after the menopause. N Engl J Med. 1984;310:1694–6.
- National Library of Medicine (US). Genetic Home Reference [Internet]. COL1A1 gene; [reviewed November 2017; cited January 2018]. Available from: https://ghr.nlm.nih.gov/gene/COL1A1
- Youngblom E, Murray ML, Byers PH. (2016). Current Practices and the provider perspectives on inconclusive genetic test results for osteogenesis imperfecta in children with unexplained fractures: ELSI implications. Journal of Law, Medicine, and Ethics, 44:514-519.
- Morgan JA, Marcus PS. (2010). Prenatal diagnosis and management of intrauterine fracture. Obstetrical and Gynecological Survey, 65(4).
- Ablin DS, Greenspan A, Reinhart M, Grix A. (1990). Differentiation of child abuse from osteogenesis imperfecta. AJR Am J Roentgenol, 154:1035–46.
- Marlowe A, Pepin MG, Byers PH. (2002). Testing for osteogenesis imperfecta in cases of suspected non- accidental injury. J Med Genet, 39:382–6
- Golshani KR, Ludwig MR, Cohn PL, Kruse R. (2016). Osteogenesis Imperfecta. Delaware Medical Journal, 88(6).
- Chagas CEA, Roque JP, Peters BSE, Lazaretti-Castro M, Martini LA. (2012). Do patients with osteogenesis imperfecta need individualized nutritional support? Nutrition, 28(2).
- Dogba MJ, Bedos C, Durigova M, Montpetit K, Wong T, Glorieux FH, Rauch F. (2013). The impact of severe osteogenesis imperfecta on
the lives of young patients and their parents – a qualitative analysis. BMC Pediatrics, 13(153).
- Dahan-Oliel N, Oliel S, Tsimicalis A, Montpetit K, Rauch F, Dogba MJ. (2015). Quality of Life in Osteogenesis Imperfecta: A Mixed-Methods Systematic Review. American Journal of Medical Genetics, 170(1).
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 National Library of Medicine (US). Genetics Home Reference [Internet]. Osteogenesis imperfecta; [reviewed April 2013; cited January 2018]. Available from: https://ghr.nlm.nih.gov/condition/osteogenesis-imperfecta
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 Steiner RD, Adsit J, Basel D. COL1A1/2-Related Osteogenesis Imperfecta. 2005 Jan 28 [Updated 2013 Feb 14]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1295/
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Forlino A, & Marini JC. (2016). Osteogenesis imperfecta. The Lancet, 387(10028), 1657-1671
- ↑ Paterson CR, McAllion S, Stellman JL. Osteogenesis imperfecta after the menopause. N Engl J Med. 1984;310:1694–6.
- ↑ National Library of Medicine (US). Genetic Home Reference [Internet]. COL1A1 gene; [reviewed November 2017; cited January 2018]. Available from: https://ghr.nlm.nih.gov/gene/COL1A1
- ↑ Youngblom E, Murray ML, Byers PH. (2016). Current Practices and the provider perspectives on inconclusive genetic test results for osteogenesis imperfecta in children with unexplained fractures: ELSI implications. Journal of Law, Medicine, and Ethics, 44:514-519.
- ↑ 7.0 7.1 7.2 Morgan JA, Marcus PS. (2010). Prenatal diagnosis and management of intrauterine fracture. Obstetrical and Gynecological Survey, 65(4).
- ↑ Ablin DS, Greenspan A, Reinhart M, Grix A. (1990). Differentiation of child abuse from osteogenesis imperfecta. AJR Am J Roentgenol, 154:1035–46.
- ↑ Marlowe A, Pepin MG, Byers PH. (2002). Testing for osteogenesis imperfecta in cases of suspected non- accidental injury. J Med Genet, 39:382–6
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Golshani KR, Ludwig MR, Cohn PL, Kruse R. (2016). Osteogenesis Imperfecta. Delaware Medical Journal, 88(6).
- ↑ Chagas CEA, Roque JP, Peters BSE, Lazaretti-Castro M, Martini LA. (2012). Do patients with osteogenesis imperfecta need individualized nutritional support? Nutrition, 28(2).
- ↑ 12.0 12.1 12.2 12.3 Dogba MJ, Bedos C, Durigova M, Montpetit K, Wong T, Glorieux FH, Rauch F. (2013). The impact of severe osteogenesis imperfecta on the lives of young patients and their parents – a qualitative analysis. BMC Pediatrics, 13(153).
- ↑ 13.0 13.1 13.2 13.3 Dahan-Oliel N, Oliel S, Tsimicalis A, Montpetit K, Rauch F, Dogba MJ. (2015). Quality of Life in Osteogenesis Imperfecta: A Mixed-Methods Systematic Review. American Journal of Medical Genetics, 170(1).