Course:MEDG550/Student Activities/Long QT
Long QT (LQT) Syndrome is a condition where the heart takes longer to recharge or prepare for the next heartbeat. The time it takes the heart to recharge is called the QT interval. In many cases, a hospital imaging tool, called an electrocardiogram (ECG), can measure the QT interval. The longer the QT interval, the longer it is taking the heart to recharge. In some cases, the delay in recharging confuses the heart and leads to very irregular heart beats (arrhythmia) which can be life-threatening [1].
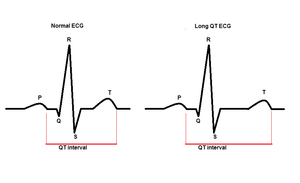
The signs and symptoms of LQT can be different between each person who has it. For example, many people with LQT do not experience any signs or have any symptoms of the condition. Others may experience fainting, or more seriously, have irregular heart beats that lead to death (cardiac arrest) [2]. Many people with LQT syndrome take medication and/or pursue other management methods to help reduce their chances of fainting and cardiac arrest[1].
LQT may be passed on from parent to child (inherited), or it may develop on its own. This document will focus on the inherited form of long QT, which has a population frequency of 1 in 2,500 individuals [3].
Specific Changes in the Heart
In order to have blood flow to all areas inside the body, the heart goes through a cycle which is controlled by electricity. This cycle is simple: the heart pumps blood all over the body, and then fills up with blood (recharges) before pumping again. In individuals with Long QT syndrome, the heart takes longer than it should to fill up. While its taking its time, areas of the body are not getting the blood they need, so they do not work the way they should. For example, if the brain is not getting blood at the right time, a person might feel light-headed and faint. Further, when the heart is not pumping at the right time, the electrical activity in the heart can become confused and go haywire. In these cases, the heart may pump in a very unusual manner (arrhythmia) and/or may stop altogether (cardiac arrest).
Channels in the heart
In order for the heart to work properly, it relies on specific "heart foods". These foods come in the form of molecules such as Sodium, Potassium, Calcium, and others. The specific balance of these molecules going inside and outside the heart allow it to pump, recharge, and have normal electrical activity. See genetics for more information about channels in individuals with LQT.
Signs and Symptoms
Many people with LQT show no signs or symptoms of the condition. For those who do show signs, these may begin in infancy through to middle-age, although it is most common to see symptoms in people from their pre-teens through to their 20s [4]. The most common symptom is fainting (syncope), which typically occurs suddenly and without warning. Cardiac arrest and sudden death are also symptoms of LQT, although these are more rare [4]. Sudden cardiac death occurs in approximately 4% of people with LQT [1].
These cardiac events may be triggered by emotional or physical distress, or startling noises like alarm clocks [1].
Diagnosis
The diagnosis of LQT can often come up when an individual has an unexplained fainting episode, which then initiates further cardiac investigations for a number of conditions. Another route that a diagnosis may arise is when investigations are initiated by a family history of sudden unexplained death or fainting. However, many people do not receive a diagnosis because they do not have any indications. Methods of diagnosis include:
Electrocardiogram (ECG) - A test which detects and records electrical activity in the heart. It would be able to show a long QT interval, and this test can be the first clue towards reaching the diagnosis. A QT of over 470 milliseconds in males, and 480 milliseconds in females, indicates a strong likelihood for LQT.
Holter monitor - This device can measure electrical activity from the heart for a longer period of time than the ECG, up to 48 hours. This is useful if an individual has cardiac changes for short periods throughout the day, and may not occur while being monitored by ECG.
Stress Test - Many individuals only have cardiac changes during physical activity or when excited. A stress test allows for the heart to be monitored during an activity which like running, or stress, when the heart is beating harder and faster than at a normal state [5].
Genetic Testing - Is used to confirm a diagnosis of an individual that has a long QT interval. The testing may provide a clearer picture as to the most effective treatment to use. Testing may also lead to screening of other family members to identify additional individuals who may be at risk.
Autopsy - Sadly, some cases of LQT are only diagnosed after there has been sudden unexplained death of an individual. An autopsy may help identify the cause.
Treatment
There are many different treatments available for those with long QT. Those offered depend several factors including the degree of risk for sudden death and medication tolerance.
Medications
Depending on the specific diagnosis, a specialized heart doctor (cardiologist) can help determine if and which medications an individual with LQT should take.
Beta-Blockers - a tablet-type of medication that helps prevent an irregular heart beat (arrhythmia). This medication can also be used along side of other treatments [6]
Potassium Channel Openers - this is a tablet-type medication that targets potassium channels in the heart. Some examples of this are: Diazoxide and Nicorandil.
Potassium Supplements - An oral supplement to increase potassium in body/heart. It can be found in many foods, such as bananas, or potatoes, or in tablet form.

Sodium Channel Blockers - a tablet-type of medication that slows the sodium going into the cells of the heart by two routes. The first is by binding to the sodium channel on the outside of the cells, and the second is by binding the sodium channel from the inside of the cell. This can control an irregular heartbeat, like that seen in LQT.
Calcium Channel Blockers - a tablet-type of medication that slows the calcium intake in the heart, and can then control irregular heartbeats, such as a long QT interval. Examples would be Nisoldipine (Sular) or Amlodipine (Norvasc)
Implantable Cardioverter Defibrillator (ICD) - a device that is surgically placed into the heart or abdomen, and control arrhythmias by using electrical pulses. This treatment option is commonly used if the patient is responding poorly to beta-blockers.
Left Cardiac Sympathetic Denervation (LCSD)- surgery in which specific nerves in the chest are removed. This surgery significantly reduces the risk of sudden death, and is usually offered only to individuals who are at risk of sudden death, do not tolerate their medications or have fainting spells despite medications. Individuals with an implanted ICD may also receive this surgery, which may reduce the frequency of ICD shocks.
Drugs and Circumstances to Avoid
In addition to the treatments listed above, there are drugs and circumstances that individuals with LQT can avoid to further decrease their risk of an arrhythmic event. Not all of these changes will be useful for all individuals with LQT, so it is helpful to discuss these suggestions with a health professional.
Drugs that prolong the QT interval - It is very important for all patients diagnosed with LQT to avoid certain drugs that can prolong the QT interval. A list of drugs to avoid is available at www.qtdrugs.org.
Exercise and sports - While physical activity can be beneficial for overall health, individuals with LQT are advised to avoid intense exercise or competitive sports. Swimming alone should also be avoided.
Startling noises and stressful situations - Loud and/or sudden noises are known to be a trigger of arrhythmia for some individuals with LQT. These risks can be reduced by turning down devices such as alarm clocks and phones, and eliminating other alarming sounds when possible. Emotional stress can also be a trigger of arrhythmia for some individuals with LQT, and should be avoided. Examples of emotionally-stressfull events include amusement park rides and jumping into cold water[4].
Genetics
The information our bodies use to determine what our our heart looks like, as well as how it pumps and recharges, is found on structures called “genes”. Individuals have two copies of each gene - one is passed on from mother and one from father.
The information on each gene is read by our body and turned into a product. For example, a gene may say “recharge” in which case the person will have their heart muscles recharge. With a spelling mistake, this word now reads “rechange”. In this case, the meaning is lost and may lead to a change in the way an individual's heart may recharge. Please see "Specific genetic changes" for a list of genes that are changed in specific forms of LQT.

Passing LQT from parent to child (Inheritance): Autosomal Dominant
LQT syndrome is typically inherited in an autosomal dominant manner (see figure). This means that an individual has one copy of their "recharging" genes not working the way it should. The second copy is usually working properly, but not enough to help take on the full role. When a person with LQT syndrome has children, they have a 50% chance of passing along the non-working copy that can cause LQT to their children.
Specific Genetic Changes
There are multiple types of LQT, with each type having a gene associated with it. Usually a single change occurs in the gene; however more severe forms have been reported with large pieces of gene missing or duplicated. The genetic testing for the following genes allows for the identification of potentially unique triggers of events, responses to the treatment or severity of the condition. Certain genetic changes have been associated with specific physiological changes, which is why genetic testing may help identify which treatments may work better than others for a particular individual.
LQT1 - The most common type of LQT, accounting for ~50% of cases. It is associated with changes in the gene KCNQ1. This change leads to the slowing of the potassium channel in the heart muscle, which in turn results in the longer QT interval. The arrhythmic events for this type are triggered by vigorous exercise, particularly swimming, as well as emotional stress.[7] Treatment with B-blockers shows a great improvement in the interval. Hearing loss may also be present.
LQT2 - The second most common type of LQT, accounting for ~40% and caused by changes in the KCNH2 gene. This gene is similar to KCNQ1 in that it also affects the potassium channels. Abnormal heart rhythms in individuals with LQT2 are triggered by startling conditions, like loud noises as well as emotional arousal.[7] Exercise and sleep may also lead to dangerous heart rhythms in individuals with LQT2.[7]
LQT3 - This type of LQT only accounts for 5-10% of the cases, and are caused by changes in the SCN5A gene. Changes in this gene affect the sodium channels, which then cause the repolarization of the heart to slow, lengthening the QT interval. Abnormal heart rhythms in individuals with LQT3 are typically triggered at rest or during sleep when the heart is beating a lower rate.[7]
The other types of LQT account for <5% of the LQT cases [8]
| LQT Type | Associated Gene |
|---|---|
| LQT4 | ANK2 |
| LQT5 | KCNE1 |
| LQT6 | KCNE2 |
| LQT7 | KCNJ2 |
| LQT8 | CACNA1C |
| LQT9 | CAV3 |
| LQT10 | SCN4B |
| LQT11 | AKAP9 |
| LQT12 | SNTA1 |
| LQT13 | KCNJ5 |
Differences in Symptom Severity
Predicting the types and severity of LQT as well as the exact age at which someone may develop LQT is difficult because:
- More than one gene can have a change/spelling mistake.
- Specific changes/spelling mistakes can happen at different places on a gene. Depending on where the change happens it can have a different effect on how the heart works.
- Genes interact with other genes. When some genes have a spelling mistake, interactions with other genes (modifier genes) may make the symptoms even more severe.
Unfortunately, science has not yet determined every gene and every change involved in PCD.
Individuals with no Symptoms
- Penetrance - Even within families who carry the same genetic change. Not everyone who inherits a genetic change that is known to cause LQT will have the condition. This is referred to as incomplete penetrance. Approximately 40% of individuals with a known genetic change do not show any symptoms [9].
Long QT (Syndromic)
LQT syndrome can happen on its own or it can be part of a larger condition/syndrome. For example, individuals with Romano-Ward syndrome and Jervell and Lange-Nielsen syndrome have LQT along with other health concerns. Romano-Ward syndrome is a clinical term that describes the autosomal dominant form of Long QT, where individuals do not have hearing loss. Jervell and Lange-Nielsen syndrome describes the autosomal recessive form of Long QT, characterized by Long QT symptoms and bilateral congenital hearing loss. Only the KCNQ1 and KCNE1 genes have been implicated with this subtype of Long QT.
Genetic Counselling Considerations
Prenatal diagnosis - Prenatal testing is available for LQT if the familial genetic change is known. It can be done with chorionic villi sampling (CVS), amniocentesis, or pre-implantation diagnosis.
Genetic testing - Genetic testing can be helpful for people who do not have a clear clinical diagnosis of LQT to confirm a diagnosis of LQT in them. Testing can also be useful for people who already have a clear diagnosis of LQT. If a genetic cause for the condition is found, then asymptomatic family members could be offered genetic testing for the familial variant to identify those who are at risk. In cases where there is a clear clinical diagnosis of LQT, a genetic explanation is found about 75% of the time [3].
Genetic counsellors are valuable resources who can help explain what LQT may mean for you and your family. To find a genetic counsellor near you, go to Find a Clinic (Canada) or Find a Genetic Counselor (USA).
Psychological Considerations: Living with long QT syndrome may impact someone both psychologically and emotionally. It is common for individuals with long QT syndrome to experience heart-focused anxiety and fear due to an increased risk of experiencing a serious heart-related event, like a sudden cardiac arrest.[10] This anxiety may lead to individuals with long QT becoming pre-occupied with or fixated on heart-related sensations, like murmurs.[10] These feelings of anxiety and concern may be heightened in individuals with long QT who have experienced a sudden cardiac arrest in their past.[11] Additionally, a diagnosis of long QT syndrome may lead to challenges with lifestyle adaptations. It is advised that some individuals with long QT syndrome do not participate in competitive sports or highly strenuous activities as exercise can trigger irregular heart rhythms.[12] Athletes or individuals who participate in rigorous physical activity as part of their daily routine may find it difficult to accept and adjust to exercise restrictions.[12] Some athletes may experience a loss of identity as they may have to give up a sport or activity that meaningfully contributed to their life for many years. Further, a diagnosis of long QT syndrome may affect the whole family. It is normal for parents of children with long QT to experience prolonged emotional distress and worry over their child’s health due to the possibility that their child could unexpectedly experience a life-threatening cardiac arrest.[13] This could lead to feelings of uncertainty and a sense of loss of control over the future. Although these are common emotions that someone may experience in response to being diagnosed or living with long QT syndrome, everyone’s experience with long QT syndrome may be different. It is important to know that there are supports available for individuals and families living with long QT syndrome. Speaking with a genetic counsellor or another healthcare professional about any psychological concerns can help ensure that individuals living with this condition are referred to appropriate support programs in their community.
Prevalence and Population Frequencies
The prevalence of LQT is 1/3000-1/7000 individuals [4]. LQT occurs in individuals of all ethnic backgrounds, although it may occur less frequently in individuals of African descent. Other populations appear to have a much higher frequency of certain LQT-causing changes.
For instance, a particular KCNQ1 change has been found in Finnish families with LQT, which has not been seen in other populations. This accounts for up to 30% of Finnish cases, and is thought to be the result of a "founder effect" [14]. A founder effect occurs when a small number of individuals (founders) expands to form a new, larger population. A genetic change present in one or more of the founders may occur at a high frequency in individuals descended from this small gene pool.
A founder effect is also thought to have occurred in the Gitxsan First Nations community in Northern BC, in which the prevalence of LQT is 1/250 or higher [15]. A novel alteration in the KCNQ1 gene has been found to be a common change in this community, and a screening strategy is being implemented.
Patient Resources
Living with, or having a family member who is living with or passed away from LQT may be extremely challenging. Support groups may provide further information on the condition and tips for management. They are an excellent source for support, allowing families to interact locally and globally.
BC Inherited Arrhythmia Program
References
- ↑ 1.0 1.1 1.2 1.3 Firth, H.V. & Hurst, J.A. (2005). Oxford Desk Reference Clinical Genetics. Oxford University Press. Pages 378-379
- ↑ Morita, H., Wu, J., & Zipes, D. P. (2008). The QT syndromes: long and short. Lancet. 372:750 63 http://www.ncbi.nlm.nih.gov/pubmed/18761222
- ↑ 3.0 3.1 Gollob, M. H. (2011). Recommendations for the use of genetic testing in the clinical evaluation of inherited cardiac arrhythmias associated with sudden cardiac death: canadian cardiovascular society/Canadian heart rhythm society joint position paper. Canadian Journal of Cardiology 27: 232-245 http://download.journals.elsevierhealth.com/pdfs/journals/0828-282X/PIIS0828282X10000942.pdf
- ↑ 4.0 4.1 4.2 4.3 Alders M, Mannens MMAM. Romano-Ward Syndrome. 2003 Feb 20 [Updated 2012 May 31]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2015. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1129/
- ↑ http://www.sads.org/SADS/media/pdf/Long-QT--3-2011.pdf
- ↑ Viskin, S., Halkin, A. (2009). Treating the long-QT syndrome in the era of implantable defibrillators. Circulation. 119: 204-206 http://circ.ahajournals.org/content/119/2/204.full.pdf+html
- ↑ 7.0 7.1 7.2 7.3 Alders M, Bikker H, Christiaans I. Long QT Syndrome. 2003 Feb 20 [Updated 2018 Feb 8]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1129/
- ↑ Udo, E.O., Baars, H.F., Winter, J.B., Wilde, A.A.M. (2007). Not just any ICD device in patients with long-QT syndrome. Neth Heart J. 15: 418-421 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2213451/
- ↑ Aatre, R. D., Day, S. M. (2011). Psychological issues in genetic testing for inherited cardiovascular diseases. Circulation Cardiovascular Genetics. 4: 81-90 http://circgenetics.ahajournals.org/content/4/1/81.full.pdf+html
- ↑ 10.0 10.1 Hamang, A., Eide, G. E., Rokne, B., Nordin, K., & Øyen, N. (2011). General anxiety, depression, and physical health in relation to symptoms of heart-focused anxiety-a cross sectional study among patients living with the risk of serious arrhythmias and sudden cardiac death. Health and quality of life outcomes, 9(1), 1-10.
- ↑ Rosman, L., Whited, A., Lampert, R., Mosesso, V. N., Lawless, C., & Sears, S. F. (2015). Cardiac anxiety after sudden cardiac arrest: Severity, predictors and clinical implications. International journal of cardiology, 181, 73-76.
- ↑ 12.0 12.1 Kapetanopoulos, A., Kluger, J., Maron, B. J., & Thompson, P. D. (2006). The congenital long QT syndrome and implications for young athletes. Medicine and science in sports and exercise, 38(5), 816-825.
- ↑ Hendriks, K. S., Grosfeld, F. J. M., Van Tintelen, J. P., Van Langen, I. M., Wilde, A. A. M., Van Den Bout, J., & Ten Kroode, H. F. J. (2005). Can parents adjust to the idea that their child is at risk for a sudden death?: Psychological impact of risk for long QT syndrome. American Journal of Medical Genetics Part A, 138(2), 107-112.
- ↑ Piippo K, Swan H, Pasternack M, Chapman H, Paavonen K, Viitasalo M, Toivonen L, Kontula K. (2001). A founder change of the potassium channel KCNQ1 in long QT syndrome: implications for estimation of disease prevalence and molecular diagnostics. J Am Coll Cardiol. 37(2):562-8.
- ↑ Arbour L, Rezazadeh S, Eldstrom J, Weget-Simms G, Rupps R, Dyer Z, Tibbits G, Accili E, Casey B, Kmetic A, Sanatani S, Fedida D. A KCNQ1 V205M missense variant causes a high rate of long QT syndrome in a First Nations community of northern British Columbia: a community-based approach to understanding the impact. (2008). Genetics in Medicine. 10(7):545.