Course:MEDG550/Student Activities/HCM
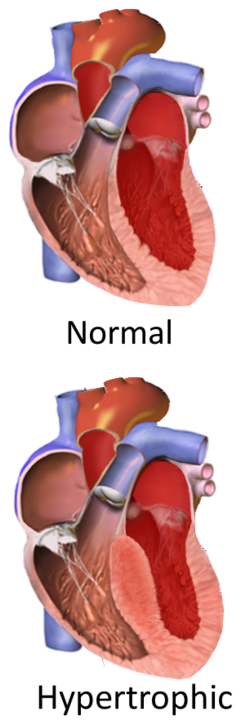
Hypertrophic cardiomyopathy (HCM) is a heart condition where a part of the heart muscle (myocardium) becomes thickened (hypertrophied), which can sometimes cause dangerous heart rhythms (also known as arrhythmias)[1]. Thickening most commonly occurs in the lower chamber on the left side of the heart (left ventricle)[1]. This thickening can then affect the heart's ability to pump effectively, causing abnormal heart rhythms that can result in heart failure and sudden cardiac death[1].
Clinical Features
HCM looks different for everybody, even for people within the same family. Many people with HCM may never develop signs or symptoms of the condition, while others experience symptoms beginning in their teens or early twenty's. The severity of symptoms ranges from mild to severe. In rare cases, sudden cardiac arrest or sudden cardiac death can be the first presentation of the condition.[1][2]
Common symptoms of HCM include:
- Shortness of breath
- Chest pain
- Lightheadedness
- Exercise intolerance
- Skipping or fluttering sensations in the chest (palpitations)
- Sudden loss of consciousness or fainting (syncope)
Diagnosis and Classification
HCM is mainly caused by the heart cells enlarging and branching into odd shapes, which creates space between them for scar tissue to build. Between the cells being physically larger, and the scar tissue forming between them, the wall of the heart grows over time [3]. HCM is diagnosed based on this thickening, which can be identified by cardiac imaging techniques, such as echocardiogram or cardiac MRI (see below)[2]. A thickness of 15 mm or more, or of 13 mm or more with a family history, is diagnostic of HCM[2][4].
- Echocardiogram – An ultrasound of the heart to identify the thickness of the heart muscle and the area of the heart where the thickening occurs.
- Cardiac magnetic resonance imaging (CMR) – An MRI of the heart to assess the function and structure fo the heart with a better resolution than an echocardiogram. Individuals with electronic heart devices like pacemakers and defibrillators may not be eligible for CMRs due to safety concerns.
- Autopsy – Unfortunately, sometimes HCM is only diagnosed after an individual has suffered a sudden cardiac death. Autopsy results may show thickening of the heart muscle.
HCM can be classified as obstructive or non-obstructive using the above cardiac imaging techniques[4]:
- Hypertrophic obstructive cardiomyopathy (HOCM) – Thickening of the heart muscle causes obstruction of the outflow of blood from the lower left chamber of the heart.
- Non-obstructive HCM (also known as Apical HCM) – Thickening of the heart muscle occurs at the lower tip of the left chamber and does not cause obstruction of the outflow of blood.
The differential diagnoses of HCM includes acquired or secondary left ventricular hypertrophy (LVH) and syndromic HCM[4]:
- Secondary LVH – Increased thickness of the lower left chamber of the heart due to other health reasons such as chronic high blood pressure, narrowing of the heart vessels or valves (aortic stenosis), or Athlete's heart. Secondary LVH is distinct from HCM because there are other biological explanations for the heart muscle thickening.
- Syndromic HCM – Conditions that have increased thickness of the lower left chamber of the heart along with other clinical features. These diseases include Danon disease, Fabry disease, Friedreich's ataxia, glycogen storage diseases, hereditary transthyretin amyloidosis, and other RASopathies.
Management
There is currently no cure for HCM but there are medical management options for at-risk individuals and those who have signs and symptoms of HCM.
- Surveillance and screening – Asymptomatic individuals with a family history of HCM are recommended to have cardiac screening every 12-18 months (from ages 12-18) or at least every five years (after the age of 18)[2][4]. Cardiac screening involves an echocardiogram to assess the thickness of the heart muscle and an electrocardiogram (ECG) that records the electrical activity of the heart. Because HCM can cause abnormal heart rhythms, ECGs are used to monitor possible arrhythmias that may develop. Individuals in competitive athletics should be monitored more often and screened for risk factors of sudden cardiac death[2]. Asymptomatic individuals should also be aware of the symptoms associated with HCM, and self monitor at all times.
- Medications – Primarily aims to relieve symptoms of HCM and control abnormal heart rhythms. Beta-blockers are commonly prescribed to lower the heart rate (by reducing the effect of adrenaline on the heart) and decrease the chance of developing abnormal heart rhythms[2].
- Surgical septal myectomy – An operation that is done to reduce symptoms in severely affected people with obstructive HCM[2]. It involves the surgical removal of the thickened heart muscle to decrease obstruction and improve blood flow.
- Alcohol septal ablation – A non-surgical technique that uses a catheter to deliver an injection of alcohol into the heart muscle to destroy some of the thickened muscle and replace it with thinner scar tissue[2]. This technique is used to relieve symptoms in individuals with obstructive HCM and improve blood flow.
- Implantable cardioverter-defibrillator (ICD) – A device that is implanted in the body to monitor the heart rhythm and deliver shocks in the event of a dangerous heart rhythm to reset the heart's pacing[2]. ICDs are more commonly implanted in individuals at risk for sudden cardiac death.
- Heart transplantation – An option for individuals that have exhausted all other forms of treatment or those who have progressed to end-stage heart failure[2].
Genetics
In many cases, the underlying cause of HCM is a small variation in a persons DNA. DNA is genetic material, which is essentially an instruction manual for all bodily functions. DNA is further divided into genes, which can be thought of as chapters in the instruction manual. Each gene has a very specific function, and when there is a change made to it, like a spelling mistake in the instructions, the gene no longer works properly. HCM is known to be caused by changes in a handful of genes that are important for regulating the structure and function of the heart. In most cases of HCM, changes are found in genes that act as instructions for creating the part of heart muscle called the sarcomere. Sarcomere's are like tiny rubber bands that stretch out to relax and pull back together to contract. For people with spelling mistakes in these genes, the rubber bands are made too thick and strong, so over time, the heart muscle gets stiff and cannot pump properly. [5]
There are at least 9 genes that are associated with HCM and more than 1400 changes in these genes have been identified[6]. Changes in these genes are found in 50-60% of individuals with a family history of HCM and 20-30% of individuals without a family history of HCM[4].
| Gene Name (Symbol) | Prevalence[6][4] |
|---|---|
| Myosin Binding Protein C, Cardiac (MYBPC3) | 50% |
| Myosin Heavy Chain 7 (MYH7) | 33% |
| Troponin I3 (TNNI3) | 5% |
| Troponin T2, Cardiac Type (TNNT2) | 4% |
| Actin Alpha Cardiac Muscle 1 (ACTC1) | <3% |
| Myosin Light Chain 2 (MYL2) | <3% |
| Myosin Light Chain 3 (MYL3) | <3% |
| Tropomyosin 1 (TPM1) | <3% |
| Phospholamban (PLN) | <3% |
There are many other genes that have been associated with HCM, but the evidence that they are disease causing is moderate to weak. [4]
Prevalence
HCM affects an estimated 1 in 500 people worldwide in the general population[7]. HCM affects both men and women equally and people of all ages[7].
Inheritance

Autosomal Dominant
Human DNA is made up of 46 chromosomes, that are matched into 23 pairs. In each pair, one copy of the chromosome is inherited from mom, and the other from dad. in this way, we pass down our DNA through the generations, and share much of the same DNA with our close relatives. HCM is typically inherited in an autosomal dominant manner, which means that a person only needs a change in one copy of the gene to be at increased risk of developing HCM. This individual then has a 50% of passing on that same gene change to all of their children.[4]
First-degree relatives (parents, children, and siblings) of an affected individual with an identified gene change are also at 50% risk of carrying the same gene change and are recommended to receive cardiac screening. First-degree relatives are also eligible for genetic testing (see below) for the specific gene change identified in the affected individual.
In a small number of people, the gene change occurs early on in conception, and was not inherited from a parent. This is called a de novo variant.
Genetic Counselling
A genetic counsellor is a genetics expert that can help individuals and families learn about their genetic testing options for HCM. Genetic counsellors and cardiologists work together to collect and assess the individual's family and medical history and help facilitate decision making regarding appropriate genetic testing and treatment options. Genetic counsellors can also provide resources and education for individuals affected with HCM. Common topics that are addressed within a counselling session are things such as genetic discrimination, sharing the news with family members, lifestyle changes, and family planning.[3][8]
Genetic Testing
Because HCM is diagnosed using cardiac imaging techniques, genetic testing is primarily used as a clinical tool for identifying at-risk first-degree relatives of an affected individual[9]. First-degree relatives who test negative for the familial gene change are not at increased risk of developing HCM and can be discharged from cardiac monitoring[9]. Genetic testing results do not affect the medical management of the affected individual and is not recommended for the purpose of risk stratification and treatment decisions[9]. Genetic testing is also not recommended for the purpose of confirming the diagnosis if there are no other at-risk first-degree relatives.[9]
Genetic testing involves providing a blood or a saliva sample which is analyzed for changes in the genes associated with HCM. When possible, testing is initiated in the most affected family member. Genetic testing can involve a panel that looks at many HCM-associated genes and there are three possible results from this type of genetic test:
- A positive result means a disease-causing or likely disease-causing change was found in one of the HCM-associated genes. Approximately 50-60% of HCM patients with a family history will test positive. Targeted genetic testing can then be offered to first-degree relatives to look specifically for the gene change found in the affected individual. The results of targeted genetic testing will either be positive or negative for the specific gene change in the family.
- A negative result means no disease-causing or likely disease-causing change was found in any of the HCM-associated genes. A negative result does not rule out the diagnosis for the affected individual and does not change their medical management. An affected individual could test negative and still have an inherited form of HCM because the available genetic tests are not exhaustive of all genes that could contribute to HCM. A negative result means that genetic testing is not available for first-degree relatives but they should undergo cardiac screening.
- A variant of uncertain significance (VUS) means that a change was found in an HCM-associated gene, but it is unknown at this time whether or not this gene change is disease-causing. Individuals with a VUS and their first-degree relatives are recommended to be followed by a cardiologist and undergo cardiac screening. In most circumstances, genetic testing is not available for first-degree relatives of individuals with a VUS, unless there is sufficient evidence that the VUS is likely to be explanatory for the individual's HCM in the context of their family and medical history.
Living with HCM
Learning that you are at risk of developing HCM can be very difficult news. You might feel that your heart is perfectly healthy because it has never caused you pain, and therefore, receiving a genetic diagnosis can feel very abrupt. If you enjoy physical exercise, being advised to change your lifestyle can be devastating, and you may even experience feelings of denial. These are all normal emotions to experience when learning this shocking news. Rest assured, having an early diagnosis can be thought of as a positive thing. It means that you and your family members will have regular cardiac screening to keep an eye on the thickness of the heart muscle, and immediate access to medications like beta blockers if your heart rhythm becomes abnormal. Early intervention will ensure that you and your family members are protected from life threatening events such as sudden cardiac arrest.
Patient Resources
For more information about HCM, visit:
For patient support groups, visit:
- Hypertrophic Cardiomyopathy Association
- The Canadian Sudden Arrhythmia Death Syndromes (SADS) Foundation
To find a genetics specialist, please visit www.cagc-accg.ca (Canada) or www.nsgc.org (United States).
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Maron, B. J., & Maron, M. S. (2013). Hypertrophic Cardiomyopathy. The Lancet, 381, 242-255. doi: 10.1016/S0140-6736(12)60397-3.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 Gersh, B. J., Maron, B. J., Bonow, R. O., Dearani, J. A., Fifer, M. A., Link, M. S., ... Yancy, C. W. (2011). 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation, 124, 2761-2796. doi: 10.1161/CIR.0b013e318223e230.
- ↑ 3.0 3.1 Basit H, Alahmadi MH, Rout P, et al. Hypertrophic Cardiomyopathy. [Updated 2024 Jun 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430788/
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Cirino, A. L., & Ho, C. (2008). Hypertrophic Cardiomyopathy Overview. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1768/.
- ↑ Wang Z, Grange M, Wagner T, Kho AL, Gautel M, Raunser S. The molecular basis for sarcomere organization in vertebrate skeletal muscle. Cell. 2021 Apr 15;184(8):2135-2150.e13. doi: 10.1016/j.cell.2021.02.047. Epub 2021 Mar 24. PMID: 33765442; PMCID: PMC8054911.
- ↑ 6.0 6.1 Alfares, A. A., Kelly, M. A., McDermott, G., Funke, B. H., Lebo, M. S., Baxter B. S., ... Rehm, H. L. (2015). Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: expanded panels offer limited additional sensitivity. Genetics in Medicine, 17, 880-888. doi: 10.1038/gim.2014.205.
- ↑ 7.0 7.1 Maron, B. J., Gardin, J. M., Flack, J. M., Gidding, S. S., Kurosaki, T. T., & Bild, D. E. (1995). Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation, 92, 785-789. doi: 10.1161/01.cir.92.4.785.
- ↑ Voseberg, H. P. (2000). Genetic counselling for hypertrophic cardiomyopathy: are we ready for it? Current Control Trials in Cardiovascular Medicine, 1, 41-44. doi: 10.1186/cvm-1-1-041.
- ↑ 9.0 9.1 9.2 9.3 Gollob, M. H., Blier, L., Brugada, R., Champagne, J., Chauhan, V., Connors, S., ... Woo, A. (2011). Recommendations for the Use of Genetic Testing in the Clinical Evaluation of Inherited Cardiac Arrhythmias Associated with Sudden Cardiac Death: Canadian Cardiovascular Society/Canadian Heart Rhythm Society Joint Position Paper. Canadian Journal of Cardiology, 27, 232-245. doi: 10.1016/j.cjca.2010.12.078.