Course:MEDG550/Student Activities/Fragile-X Syndrome
Fragile X syndrome (FXS) is a genetic condition that can cause varying developmental differences.[1] Individuals affected with FXS have a normal life expectancy, however, most will require support and care for their entire lives. FXS is the most common inherited form of intellectual differences
Synonyms
- Fragile site, folic acid type, rare, Fra(X)(Q27.3)
- Marker X syndrome
- Martin-Bell syndrome
Clinical Features
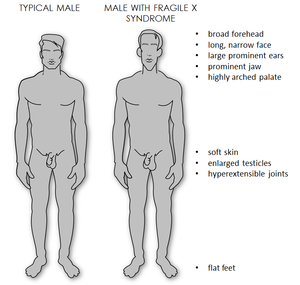
While symptom severity varies, all males, and some females, present with significant mental delay and behavioural difficulties. [2] Usually, males are more severely affected by this condition than females. [2] Most males and about half of females with FXS have characteristic physical features that become more apparent with age. These features include a long and narrow face, large ears, a prominent jaw and forehead, unusually flexible fingers, flat feet, and in males, enlarged testicles after puberty. [2]
Individuals with FXS usually exhibit delayed development of speech and language by the age of two. Men affected with FXS are nearly always characterized as having moderate intellectual disability whereas intellectual disability for women affected with FXS tends to be more mild. [3]
Children with FXS may also display anxiety or hyperactive behaviour, such as fidgeting or impulsive actions. They may also have attention deficit disorder, which includes an impaired ability to maintain attention and to focus on specific tasks. About one-third of individuals with FXS have features of autism spectrum disorders. These features affect communication and social interaction. Seizures occur in about 15% of males and about 5% of females with FXS. [3]
Inheritance
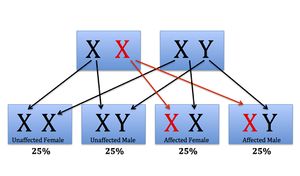
FXS is inherited in an X-linked dominant pattern. [2] Half of all offspring could inherit the X chromosome with the mutation from their mother. Males and females are equally likely to be affected.
A condition is considered X-linked if the altered, or mutated, gene that causes the disorder is located on the X chromosome. The X chromosome is one of the two sex chromosomes. Females have two X chromosomes, while males have an X and a Y chromosome. Dominant inheritance means that having one copy of an altered gene in each cell is sufficient to cause the condition. When a female has an X-linked dominant condition, such as FXS, she has inherited only one mutation on one of her X chromosomes. This is sufficient to cause the disorder, as her intact X chromosome cannot compensate for the altered X chromosome. Since males only have one X chromosome, a mutation in this chromosome causes the disorder.
Because women have two X chromosomes, females with FXS have more variable clinical manifestations than males. This is because one of the X chromosomes in females is randomly selected to be inactivated, or turned off. The proportion of cells with the active X chromosome that carries the fragile X mutation determines the severity in females. [1] A limitation of genetic testing is that the clinical manifestations of FXS in a female cannot be predicted by routine testing.
Genes

FXS is caused by a difference (i.e mutation) in a gene called Fragile-X Mental Retardation-1 (FMR1), on the X chromosome. [3] The FMR1 gene provides the instructions for making a protein called fragile X mental retardation 1 protein. This protein helps regulate the production of other proteins and plays a role in the development of synapses, which are specialized connections between nerve cells. [3] Synapses are important for healthy communication between the neveres cells.
Nearly all cases of FXS are caused by a mutation that expands part of the FMR1 gene. This expansion occurs when a small region of DNA, known as the CGG trinucleotide repeat, is repeated many times within the gene. [3] Normally, this DNA segment is repeated anywhere from 5 to 40 times. In people with FXS, the CGG segment is repeated more than 200 times. The abnormally expanded CGG segment turns off, or silences, the FMR1 gene, which prevents the gene from producing any protein. Without the gene turned on there is a shortage of the protein being made, and this loss disrupts nervous system functions and leads to the signs and symptoms of FXS. [3]
CGG Expansion Lengths & Their Implications
FMR1 gene differences are classified into four groups based on repeat length. These groups can be seen below:
Normal
Healthy copies of the FMR1 gene contain 6–44 CGG repeats. [1]
Intermediate
Intermediate or “gray zone” gene differences contain 45–54 CGG repeats. [1]
Intermediate differences do not cause FXS. These genes are "unstable" have may grow in repeat length when passed from a mother to a child.[1]
Premutation
Premutation gene differences contain 56–200 CGG repeats. [1]
Some premutation carriers, primarily males, have an increased chance of developing tremor/ataxia syndrome or psychological symptoms later in life. [1] This is called Fragile X-associated tremor/ataxia syndrome (FXTAS) and can cause motor disturbances and tremors, short-term memory loss, cognitive decline, dementia, parkinsonism, or lower-limb muscle weakness.
Female premutation carriers have an increased chance of developing premature ovarian failure (POF). POF occurs when menopause begins under the age of 40 years.[4] This can result in fertility challenges. The risk of developing POF if you are a premutation carrier is around 21%. [4]
Most people with a premutation have no intellectual differences. In some cases, individuals with a premutation have lower than normal amounts of protein. As a result, they may have mild versions of the physical features seen in FXS, such as prominent ears, and may experience challenges such as anxiety or depression. [3]
In women, the FMR1 gene premutation can grow to more than 200 CGG repeats in cells that develop into eggs. This means that women with the premutation have an increased chance of having a child with FXS.[1] In contrast, the premutation in men does not expand to more than 200 repeats in sperm. This means that men with the premutation do not have an increased risk of having a daughter with FXS. [1]
Full mutation
Full mutations contain more than 200 CGG repeats, with a range of several hundred to several thousand repeats. [1]
People with this repeat range have the typical clinical manifestations described in the Clinical Features section of this page.
Prevalence
FXS occurs in approximately 1 in 4,000 males and 1 in 8,000 females [1] and is the second most prevalent genetic cause of mental deficiency after Down syndrome. [5]
A carrier of FXS is an individual who has a number of repeats within FMR1 that exceeds the normal range, but who does not themselves display symptoms of FXS. The prevalence of women who are carriers of FXS is estimated to be about 1 in 154 in individuals without a family history of mental retardation, developmental problems, or autism. In individuals with a family history of mental retardation, developmental problems, or autism, the carrier rate is 1 in 128. [1]
Management
Currently there is no cure for FXS. Early developmental intervention and special education with individual attention, small class size, and avoiding sudden change or excessive stimulation can help in the development of children with FXS. Individualized medical management of behavioral issues can also help improve social interaction. [6] Any medical problems that arise should be discussed with a health care professional.
Options are available to help treat and manage the condition. Due to the complex nature of the condition, individuals with FXS generally have a personalized care plan, involving a multidisciplinary health care team, to help manage both the medical and developmental aspects of their condition. This may include neurologists, occupational and physical therapists, speech language therapists, and other specialists. Early intervention is recommended to maximize the abilities of individuals with FXS, and can begin within the first years of life[7].
Motor Skills
Physical therapy can help individuals with FXS to improve their mobility and muscle tone. This therapy can also help reduce the risk of additional health complications, such as dislocations or scoliosis, later in life. Some individuals may also benefit from the use of mobility devices, such as wheel chairs, walkers, or orthotics, depending on their individual needs[7].
Similarly, Occupational therapy can help to improve the fine motor skills required for tasks such as using cutlery for eating, combing hair, or writing. It is recommended that young children with FXS attend occupational therapy at least twice a week[8].
Speech therapy can help in the development of language skills. In cases where speech is very delayed a speech-language pathologist may also be able to provide options of alternative methods of communication.
Behaviour and Social Skills
Behavioural challenges faced by individuals with FXS may include hyperarousal, irritability, anxiety, and aggressive or self-injurious behaviours. Behavioural therapy can be provided to help individuals develop self-regulation and coping skills, as well as to develop strategies to avoid and manage triggers. Young children with FXS should also be evaluated for autism spectrum disorder, which typically manifests around two years of age[9].
Sensory Challenges are a common problem faced by children with FXS. They may be oversensitive to some inputs, making them uncomfortable or overwhelmed in some situations. This may present itself as behavioural issues such as picky eating, difficulty brushing teeth, poor attention, poor coping skills (such as temper tantrums), difficulty wearing some clothing, and toilet training issues. An occupational therapist may be able to provide strategies to help individuals learn to cope with distressing stimuli, or accommodations to help them avoid these situations[8].
Sleep difficulties are common in individuals with FXS. Establishing a regular sleep schedule, with a consistent and calming bedtime routine is recommended to assist with this. Medications, such as melatonin, are also available to assist with sleep if needed[8].
Medications
When behavioural interventions are not sufficient, medication is available to help control hyperactivity, aggressive or self-injurious behaviours, and anxiety. Numerous medications are also available that can effectively control the seizures that occur in some individuals with FXS[7].
Education
Children with FXS may benefit from special education support based on their individual needs. This may be an educational support person, or enrollment in a special education program.
Testing & Genetic Counselling
Testing
Testing is available to confirm the presence of a FMR1 expansion mutation in someone with suspected FXS or to identify the repeat length in their parent. DNA analysis is the method used for the diagnosis of FXS and the identification of carriers. Testing consists of evaluating the size of the CGG repeat in the FMR1 gene. Results reliably classify individuals as being within the normal, intermediate, premutation, or full mutation category. [3]
Precision of Testing
The estimated precision range in the measurement of intermediate alleles is typically reported by clinical laboratories performing FMR1 analysis. Estimates tend to be no more than ±2-3 repeats. Because of this, test results that are close to the characterization boundaries should be carefully considered. [3] For example, an intermediate allele with 55 repeats should also be considered as a potential premutation. If the repeat precision estimate is not on the laboratory report, the laboratory should be contacted in order to determine if a result should be considered as a potential premutation.
Testing Implications
All mothers of individuals with an FMR1 full mutation are considered to be obligate FMR1 premutation carriers. These women, as well as their female relatives are at increased risk for POF or FXTAS.
Women with either a premutation or a full mutation are at increased risk of having offspring with FXS, FXTAS, and POF.
Males with premutations are at increased risk for FXTAS. Males with FXTAS will not transmit their FMR1 premutation expansion to any of their sons but will transmit it to all of their daughters. Their daughters will be premutation carriers.
Testing Options
Clarification of an individual's FXS genetic status is possible when there is a family history of FXS or a related disorder. [3] Predictive testing for at-risk asymptomatic adult family members, including male relatives at risk for FXTAS and female relatives at risk for POF, requires prior identification of an expanded FMR1 allele in the family. [3]
Prenatal diagnosis or prenatal testing for at-risk pregnancies requires prior confirmation of the presence of an expanded FMR1 allele in the family. When a pregnant woman has been confirmed to have a premutation or full mutation in the FMR1 gene, prenatal testing by either chorionic villus sampling or amniocentesis is available. [1]
Preimplantation genetic diagnosis (PGD) is a prenatal testing technology involving in-vitro fertilization (IVF) techniques. Embryos are created using IVF, and are tested for FMR1 gene status prior to implantation in the mother's uterus. [3] Embryos that do not carry a full mutation are selected to be implanted. Like the other prenatal testing options, this technology requires prior confirmation of the presence of an expanded FMR1 gene in the family. In British Columbia, PGD is not covered by provincial medical insurance, but is available through private pay facilities.
Genetic Counselling
Following diagnosis by molecular confirmation and analysis of repeat size, genetic counselling can give a risk assessment to other family members. For males with premutation there is virtually no risk of having a child with a FXS. Daughters will receive an X chromosome from their fathers, but it will maintain its premutation triplet repeat size and she would be considered a carrier. Sons receive their father's Y chromosome, so there is no genetic risk to sons of a premutation carrier. Reproduction in males with the full mutation is extremely rare, but it is possible that there could be a contraction of triplet repeats and a daughter who is a premutation carrier or a daughter who receives the full mutation. [5]
Females with a premutation have a 50% chance of passing on the Fragile X gene, but the risk of having a child with Fragile X will depend on the size of the mother's premutation. Women with premutations of less than 60 repeats have a very small risk of having a child with FXS. If the repeat number is greater than 90 ("high-end permutation) the risk is in the range of 90% to 100%.
Very rarely, contraction of the trinucleotide repeat has been reported where a mother with a premutation gave birth to a daughter whose triplet repeat size was in the normal range. [3] All male offspring who receive the FMR1 mutation will have Fragile X, as will 60% of the girls. The intellectual disability in affected females is usually less severe than in the male. [5]
Genetic counsellors can help patients understand their diagnosis, their chance of having an affected child, and testing options. Consider speaking to a genetic counsellor near you to learn more about how FXS may affect your or your family.
Canada: Canadian Association of Genetic Counsellors
United States: National Society of Genetic Counselors
Patient Resources

Patient Resources and Support Groups
Fragile X Research Foundation of Canada
- The Fragile X Research Foundation of Canada is a volunteer-based organization that strives to rransform the lives of individuals with Fragile X, their families, and the networks of professionals who serve them. To achieve this mission, the foundation facilitates community education, awareness and advocacy, as well as basic and clinical research into treatments of Fragile X and it’s related conditions.
- The National Fragile X Foundation aims to serve the entire Fragile X community to live their best lives by providing the knowledge, resources, and tools. this foundation works towards their mission through prioritizing patient advocacy, education, research, and treatment.
It is common for affected families to feel a sense of isolation after receiving a rare disease diagnosis. Many find comfort in reading about the stories of other individuals affected by a similar condition, or in joining online support groups. These can be found within the resources listed above.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Chitayat, David. et al. Fragile X Testing in Obstetrics and Gynaecology in Canada. Joint SOGC-CMMG Committee Opinion. No 216, Sept 2008
- ↑ 2.0 2.1 2.2 2.3 Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: {#300624}: {9/26/2012}: URL: http://omim.org/entry/300624
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 Saul, R.A., et al. “FMR1-Related Disorders”. 1998 Jun 16 [Updated 2012 Apr 26]. In: Pagon RA, Bird TD, Dolan CR, et al., editors. GeneReviews™ [Internet]. Seattle (WA): University of Washington, Seattle; 1993-. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1384/.
- ↑ 4.0 4.1 Sherman, S.L. (2000) “Premature ovarian failure in the fragile X syndrome”. American Journal of Medical Genetics 97:3 189-194
- ↑ 5.0 5.1 5.2 McKinley Gardner, R.J., G.R. Sutherland, and L.G. Shaffer, Chromosome Abnormalities and Genetic Counseling. 4th ed. 2012, New York, New York: Oxford University Press
- ↑ Crawford, D. et al. “FMR1 and the fragile X syndrome: Human genome epidemiology review”. Genetics in Medicine 2001;3:359-371.
- ↑ 7.0 7.1 7.2 Hunter JE, Berry-Kravis E, Hipp H, et al. FMR1 Disorders. 1998 Jun 16 [Updated 2019 Nov 21]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1384/
- ↑ 8.0 8.1 8.2 Lozano R, Azarang A, Wilaisakditipakorn T, Hagerman RJ. Fragile X syndrome: A review of clinical management. Intractable Rare Dis Res. 2016;5(3):145-157. doi:10.5582/irdr.2016.01048
- ↑ Hagerman RJ, Berry-Kravis E, Hazlett HC, Bailey DB Jr, Moine H, Kooy RF, Tassone F, Gantois I, Sonenberg N, Mandel JL, Hagerman PJ. Fragile X syndrome. Nat Rev Dis Primers. 2017 Sep 29;3:17065. doi: 10.1038/nrdp.2017.65. PMID: 28960184.