Course:MEDG550/Student Activities/Duchenne and Becker Muscular Dystrophy
Muscular dystrophies are a group of inherited conditions that are characterized by muscle weakness and a loss of muscle tissue, both of which worsen over time. Muscular dystrophies may occur in childhood or adulthood.[1] There are many different types of muscular dystrophy, including Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD).
Cause of Duchenne/Becker Muscular Dystrophy

DMD and BMD are both caused by an alteration (or mutation) in the dystrophin (DMD) gene that makes a muscle protein by the same name.[2] The dystrophin gene is the longest known human gene (2.5 Mb) and is located on the short arm of the X chromosome (locus Xp21).[3] This genetic change affects the ability of muscles to make dystrophin. The dystrophin protein provides support in muscle fibers to give strength and prevent injury. Without dystrophin, muscles are unable to contract properly resulting in muscle weakness.
Genetic Changes in Duchenne/Becker Muscular Dystrophy
While DMD and BMD both result from changes in the dystrophin gene, individuals with BMD typically have a later onset or milder symptoms than individuals with DMD. This is thought to be due to the particular type of alteration in the DMD gene and how it affects dystrophin function.
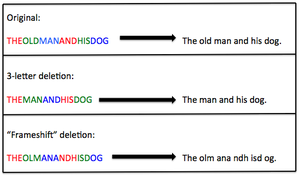
The information contained in genes is read by a 3-letter code. Most cases (60-70%) of DMD and BMD result from deletions within the dystrophin gene [3]. If the deletion is a multiple of 3, some information will be missing but the rest of the gene will still be organized properly in 3-letter codes. In this case, the dystrophin protein won't function quite as well but can still provide some support to the muscle fiber. This is the case usually seen in BMD. If the deletion is not a multiple of 3, this shifts the genetic information into different 3-letter codes which can't be read properly and don't make any sense. This is referred to as a "frameshift" mutation, and usually results in no functional protein being made from that gene. This type of deletion causes a more severe presentation and is usually seen in DMD.
Inheritance of Duchenne/Becker Muscular Dystrophy
Both DMD and BMD can be passed along through the family or can occur sporadically. DMD and BMD are known as an X-linked recessive diseases. Due to the way that they are inherited, DMD and BMD affect more males than females. This is because the gene for dystrophin is on the X chromosome. Females have two X chromosomes so even if one copy is has the alteration, the other can still produce dystrophin. Males on the other hand, only have one X chromosome and therefore if their only X chromosome has an altered dystrophin gene they will not be able to make any protein. The offspring of females who are carriers of DMD or BMD (those who have an altered dystrophin gene but usually no symptoms) have a 50% chance of inheriting the altered gene from their mothers. Since this is an inherited disorder, risks include having a family history of DMD or BMD. DMD affects 1 in 3,500 males,[4] whereas BMD affects 1 in 18,000 males.[5]

Diagnosis of Duchenne/Becker Muscular Dystrophy
DMD and BMD can be diagnosed through a DNA blood test that shows a change in the dystrophin gene. Most individuals with DMD and BMD (60-70%) loss of genetic material in the DMD gene, with a deletion of at least one exon. There is no correlation between the size of the deletion and the severity of the disease.[2] The next most common genetic changes known to cause DMD (25-35%) and BMD (10-20%) are point mutations, or single base changes that interfere with production of dystrophin protein. In 5-10% of cases, a duplication of one or more exons in the DMD gene causes DMD or BMD. Sequencing of the entire gene can be necessary to detect rare mutations or those that are unique to a single family ("private mutations"). When considering testing for females who may be carrying one of these genetic changes that could be passed to a son, it is useful to know which form of genetic change occurs in her family to ensure that her test results will be accurate.[3]
If the genetic test is normal, a diagnosis can be made by the combination of clinical findings, family history, elevated creatine kinase concentration in the blood, and muscle biopsy to identify a lack of dystrophin in the muscle cells.
Duchenne/Becker Muscular Dystrophy in Females
The majority of female carriers of mutations in the dystrophin genes will not have any of the classical signs of the either DMD or BMD.[6] Asymptomatic females include those who may have elevated CK levels with mild muscle cramping.[6] Between 2-22% of carrier females will present with a range of symptoms from mild muscle weakness to a similar clinical course of DMD.[6] Some females may also experience changes to the heart (cardiomyopathies) later in life [6]. In BMD 5-10% of female carriers may also show enlarged calves.[5]
Duchenne Muscular Dystrophy
Clinical Features
DMD is characterized by slowly progressive muscle-wasting [1]. Signs of weakness in DMD usually occur before age 5, typically between ages 2 and 4.[1] Initial symptoms include the inability to run or keep up with fellow peers and it is typical that affected individuals can only climb one step at a time.[1] The progression of weakness becomes more disabling in late childhood and many individuals with DMD are wheelchair-bound by late childhood or early teens.[1]
Management
Currently there is no cure for DMD. However, considerable progress has been made in terms of the management of DMD. Treatments aim to control symptoms to improve the quality of life for affected individuals. Steroids including prednisone, prednisolone and deflazacort have appeared to slow the disease process. [1] DMD progresses more quickly than BMD, and children generally require the use of a wheelchair for mobility before age 12. A variety of molecular therapies are currently being researched. [1]
Becker Muscular Dystrophy
Clinical Features
BMD is similar to DMD in terms of muscle wasting and weakness but age of onset is much later, usually around 12,[5] with some patients not showing any symptoms until adulthood.[1] Initial symptoms are fatigue or weakness in the thighs, trouble climbing steps, occasional calf or thigh cramps. [1] Affected individuals are usually mobile beyond the age of 15 in contrast to those with DMD. [1]
Management
Treatments aim to control symptoms to improve the quality of life for affected individuals since there is no cure for BMD. Activity is encouraged since inactivity can make the disease worse. Physical therapy may help to improve muscle strength. A variety of molecular therapies are currently being researched. [1]
Cardiac Involvement
In addition to affecting skeletal muscles, changes in the dystrophin gene can also result in heart muscle problems known as cardiomyopathies. The most common heart problem associated with BMD and DMD is dilated cardiomyopathy, in which the heart becomes enlarged and cannot pump blood as efficiently.[7] Dilated cardiomyopathy may cause symptoms such as shortness of breath, irregular heartbeat, and fatigue.[8] In some cases, dilated cardiomyopathy leads to congestive heart failure and death.[3]
Cardiomyopathy in BMD and DMD
For both BMD and DMD, heart problems usually start in the teenage years and are diagnosed around age 14 [3]. For individuals with DMD, the likelihood of developing dilated cardiomyopathy increases steadily with age, and by age 20 nearly 100% of people with DMD are affected. In contrast, only 70% of people with BMD will have a heart problem by age 20.[7] Although the progression may be slower, dilated cardiomyopathy is the most common cause of death in individuals with BMD.[3]
DMD-Associated Dilated Cardiomyopathy
Changes at specific locations within the dystrophin gene can cause heart problems without affecting other muscles in the body. These genetic changes result in a condition known as DMD-associated dilated cardiomyopathy, which is distinct from BMD and DMD.[8] Unlike BMD and DMD, individuals with DMD-associated dilated cardiomyopathy usually do not have any skeletal muscle problems such as weak legs. In males with this condition, heart problems appear early in life and are quite severe. Females carriers tend to develop heart problems later in life, and it takes longer for the problem to become severe.[8]
Genetic Counselling for Duchenne/Becker Muscular Dystrophy
For those with a family history or those living with Duchenne or Becker Muscular Dystrophy, genetic counselling can provide several benefits. A genetic counsellor can explain the cause, the symptoms of the disorder, as well as facilitate diagnostic and genetic testing. In addition, a genetic counsellor can discuss the emoional impact that Duchenne and Becker Muscular Dystrophy can have on families. It is the genetic counsellor who can help families prepare for, identify and manage the difficulties associated with having a child with DMD or BMD. The diagnostic process is often quite emotional for families. Referrals for genetic counselling can be made by a primary care provider.
Living with Duchenne/Becker Muscular Dystrophy
People living with Duchenne and Becker Muscular Dystrophy have a unique set of challenges and opportunities in their lives. Interviews with people who live with these conditions have outlined some experiences common to this patient group[9]. Ultimately, individual stories will always be just that: individual. However, the themes below give a sense of what life might be like for someone living with DMD or BMD:
Living with medical interventions
Time burden. Particularly for children with DMD, a large part of a child’s life is overrun by visits to different medical professionals (cardiologists, neurologists, respirologists, etc). For patients, the time associated with these visits can be burdensome to regular social activities. Some parents and children feel that their life is taken over by visits to the doctor[9].
Living with pain. These conditions involve living with chronic pain. Interviewers stated that they admired the ability of young people to cope with so much daily pain. In addition to daily pain, patients may have to go through particularly difficult surgeries. Both the recovery time and the surgery itself can be difficult to go through. One young man states, about his spinal surgery: “It just hurt all the time. The things that are inside of you are horrible. And the needles bruised me every time they put something in it. It was horrible.”[9]
Making healthcare decisions. In the case of DMD, as young boys become young men, they need to begin making decisions about heir healthcare. These usually involve decisions about further spinal surgery, assisted ventilation, and treatment about heart problems. At a very young age, patients are forced to deal with the incredibly difficult question of whether these treatments are worth going through.[9]
Moving to a wheelchair. Boys with DMD are wheelchair-dependent, on average, at the age of 13[9]. For some, losing the ability to walk is the most difficult change in their life. One patient described this change as more difficult than going away for university. [9]
Restricted independence
Reliance on caregivers. Because of the severe health problems associated with this condition, people are often limited in what they can do without the presence of a caregiver. Many people with DMD are dependent on their parents for most, if not all, of their lives. While some patients attend university and college, many do not. Even though they may rely on their caregivers, many of these boys and young men feel as if they cannot talk to their parents about what they might be feeling and feel the need to “protect” them from their pain.[9]
Reliance on health interventions. Many people with DMD and BMD rely on breathing interventions and wheelchairs. These machines can often be cumbersome and loud. Some described being afraid that something would go wrong with the machinery when they left the house. One person told an interviewer: “This machine's holding me back”.[9]
Finding community/peer support
Low confidence and limitations to social life. People with DMD describe finding it difficult to engage in typical social activities for their age group because of their interventions. Chest infections, spinal surgery, and ventilation usage can be physically limiting. One boy explained that having a tracheotomy affected his confidence. Another described being worried about his breathing, and not being able to make long term plans because he didn’t know whether he would feel too tired to follow through with them.[9]
Difficulty in talking to parents. For many people with DMD, their parents are an incredibly valuable source of support. However, some do not feel comfortable talking to their parents because they did not want to worry them. Some turn to other relatives or support groups.[9]
Peer support groups. The helpfulness of support groups for people with DMD tends to vary. Some find support groups incredibly helpful, saying that it is a place where they can talk about things that they cannot talk to their parents about. Coping strategies and common experiences are shared with each other in a comfortable place. One young man stated that they could “talk about a really depressing subject but have a laugh about it”. On the other hand, some individuals chose not to attend a support group because they felt that it would be difficult to see others who were in the same, or possibly a worse situation than them. Even those who found support groups to be very helpful felt that there were some drawbacks, the biggest being that it was painful to make friends who may soon pass away. One mother stated about her son with DMD: “He had several friends with muscular dystrophy at school and they're all dead”. Often at a young age, an individual then has to deal with both the loss of his friend as well as fear of his own future.[9]
Overall quality of life
Despite the numerous difficulties associated with both DMD and BMD, quality of life scores are generally still high. In one study of adults with DMD, quality of life scores were high in all categories except qualifying education, love life, and pain[10]. In a study of individuals with all types of muscular dystrophy, it was found that patients with BMD had the highest overall quality of life scores when compared to other types of muscular dystrophy[11].
Resources
- Muscular Dystrophy Campaign
- Muscular Dystrophy Canada
- Families Advocating, Connecting, Educating, and Supporting (FACES)
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Chamberlain, J.S., Rando, T.A. (2006). Duchenne Muscular Dystrophy: Advances in Therapeutics. Taylor & Francis Group. New York.
- ↑ 2.0 2.1 Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: 310200 {Date last edited}: October 3, 2012. World Wide Web URL: http://omim.org/entry/310200
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Darras BT, Miller DT, Urion DK. Dystrophinopathies. 2000 Sep 5 [Updated 2011 Nov 23]. In: Pagon RA, Adam MP, Bird TD, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2014. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1119/
- ↑ Nowak, K.J., Davies, K.E. Duchenne muscular dystrophy and dystrophin: pathogenesis and opportunities for treatment. EMBO reports, 5, 872-876.
- ↑ 5.0 5.1 5.2 Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: 300376 {Date last edited}: January 18, 2012. World Wide Web URL: http://www.omim.org/entry/300376
- ↑ 6.0 6.1 6.2 6.3 Brioschi, S., Gualani, F., Scotton, C., Armaroli, A., Bovolenta, M., Falzarano, M., ... Ferlini, A. (2012) Genetic characterization in symptomatic female DMD carriers: lack of relationship between X-inactivation, transcriptional DMD allele balancing and phenotype. BMC Medical Genetics, 13:73
- ↑ 7.0 7.1 Jefferies, J. L., Eidem, B. W., Belmont, J. W., Craigen, W. J., Ware, S. M., Fernbach, S. D., ... & Towbin, J. A. (2005). Genetic predictors and remodeling of dilated cardiomyopathy in muscular dystrophy. Circulation, 112(18), 2799-2804.
- ↑ 8.0 8.1 8.2 National Library of Medicine (US). Genetics Home Reference [Internet]. Bethesda (MD): The Library; 2015 Mar 16. DMD-associated dilated cardiomyopathy; [reviewed 2012 Feb; cited 2015 Mar 26]; [about 4 screens]. Available from: http://ghr.nlm.nih.gov/condition/dmd-associated-dilated-cardiomyopathy
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 Abbott, David (2014). "'The Things that are Inside of You are Horrible': Children and Young Men with Duchenne Muscular Dystrophy Talk about the Impact of Living with a Long-term Condition". Child Care in Practice. 21: 67–77.
- ↑ Rahbek, Jes (2005). "Adult life with Duchenne muscular dystrophy: Observations among an emerging and unforseen patient population". Pediatric Rehabilitation. 8: 17–28.
- ↑ Grootenhuis, Martha (2007). "Living with muscular dystrophy: health related quality of life consequences for children and adults". Health and quality of life outcomes. 5: 31.