Course:MEDG550/Student Activities/CMT1
Charcot-Marie-Tooth Disease (CMT), also known as hereditary motor and sensory neuropathy (HMSN) or peroneal muscular atrophy (PMA), is a family of disorders that cause neuropathy, meaning it causes damage to the nerves of the peripheral nervous system (PNS). The nerves of the PNS are those found outside of the brain and spinal cord. These nerves are made up of bundles of many nervous cells, named neurons. In the body, these neuron bundles allow our brain to communicate with the rest of the body. There are 3 main types of neurons in the PNS: 1) motor neurons - which allow us to move, 2) sensory neurons - which gives us our sense of touch, sight, smell and taste, and 3) autonomic neurons - which controls our body's automatic and involuntary functions, like maintaining a normal heart beat and our reflexes, like moving your hand away from a hot surface.
In the body of a person with CMT, the motor and sensory nerves are damaged over time. This damage prevents the PNS from working correctly, so a person affected with CMT gradually becomes weaker, looses sensation across the body, and can even result in deformities of the hands or feet.
CMT is the most commonly inherited neurological disorder, and was first described in 1886 by Drs. Jean-Martin Charcot and Pierre Marie in Paris, France, and Dr. Howard Henry Tooth in Cambridge, England.[1]
Charcot-Marie-Tooth Disease, type 1 (CMT1) is the most common type of CMT, and makes up 50-80% of all CMT cases.[2] CMT1 mostly affects the extremities, meaning that the majority of the symptoms affect the arms and legs.
What does CMT1 look like?
CMT is the most common inherited neuromuscular disorder[3]. As a group of disorders, CMT affects approximately 1 in 2500 people[1][4], while CMT1 accounts for 50-80% of all CMT cases.[2] Men and women are affected equally.
Symptoms of CMT will usually start in an individual when they are around 5-25 years old. Although most people affected by CMT1 will have symptoms by the time they are young adults, a small portion of patients will only develop symptoms in late adulthood. The timing and severity of the symptoms can vary from person to person, even within the same family.

The general features for all subtypes of CMT1 include[4][2]:
- Weakness in legs and feet, progressing to arms and hands
- Loss of touch sensation in feet, ankles, legs and hands, wrists, and arms
- Difficulty lifting the foot (foot drop)
- Curled toes (hammer toes)
- High foot arches
- Muscle loss on the lower parts of the leg, giving rise to a “stork leg” or “inverted champagne bottle” appearance
- Reduced or absent reflexes
- Hearing loss
The onset of symptoms is typically gradual, and progress slowly over the course of the person's lifetime. The symptoms are often described as painless, but this is not true for all individuals. Although changes to a person's gait, meaning how they walk, is fairly common with CMT1, fewer than 5% of individuals will require the use of a wheelchair [1].
What cause of CMT1?

A nerve cell communicates information to different parts of the body by sending electrical signals down a long, thin part of the cell called the axon. Axons are covered by the myelin sheath, which is a layer of protein and fat that wraps around the axons to keep them insulated and prevents the loss of electrical signals. It also protects the axons, and makes sure that signals are communicated through the nerves quickly and efficiently. Without an intact axon and myelin sheath, PNS nerve cells become inefficient at relaying motor or sensory information between the brain and the limbs.
CMT1 is often referred to as the "demyelinating" form of CMT, due to the fact that affected individuals will develop myelin sheath abnormalities[1][3]. As a result, the signals between the brain and limbs will sometimes get interrupted, which causes numbing or difficulties moving.
The myelin sheath abnormalities typically observed in CMT1 develop due to specific genetic changes that alter the way that myelin is formed and maintained in the body. The different subtypes of CMT1 are separated based on which specific gene, or set of instructions, are responsible for causing the abnormality in the myelin[5]. Most of these genes contain the instructions on how to assemble proteins that normally make up myelin.
Genetics
Genes, which are short stretches of DNA in our cells, contain instructions for our bodies to build proteins. These proteins are the ones who create structures within our bodies, or carry out a specific task. Each person has a specific set of instruction, and there are millions of different ways that the instructions can be written out. These variations is what makes us all unique. Sometimes though, the instruction can have a mistake. If the mistake makes it that the body can not read the instructions and form the protein properly, it is considered a disease-causing variation, or a mutation. Some mutations have very small impact on how our bodies work, whereas other mutations can have very harmful effects. It depends on what the job of the protein was supposed to be, and how much of the protein is affected by the spelling mistake.
Genes can have different kinds of disease-causing variation. These can include point mutations (which are like a typo in the instructions) deletions (which is when a section of the instruction is missing) and duplications (where there is a section of the instruction that is repeated).
Genes involved in CMT1
There are 6 subtypes of CMT1, with a different gene responsible for causing each of the subtypes[5].
PMP22 is the most common causative gene for CMT1, and is responsible for both CMT1A and CMT1E. CMT1A is the most subtype of CMT1 and of CMT overall, where there is a duplication of PMP22 that leads to overexpression of the protein to cause disease.
| Subtype | Gene Name (Symbol)[1][4] | Prevalence[2] |
|---|---|---|
| CMT1A | Peripheral myelin protein 22 kD (PMP22) | 80-90% of cases of CMT1 |
| CMT1B | Myelin protein zero (MPZ) | 10% of cases of CMT1 |
| CMT1C | Lipopolysaccharide-induced TNF factor (LITAF) | 1-2% of cases of CMT1 |
| CMT1D | Early growth response protein 2 (EGR2) | <2% of cases of CMT1 |
| CMT1E | Peripheral myelin protein 22 kD (PMP22) | <5% of cases of CMT1 |
| CMT1F | Neurofilament light polypeptide (NEFL) | <5% of cases of CMT1 |
How is CMT1 diagnosed?
Diagnoses for CMT1 are made based on a combination of clinical observations, testing, and family history.[1]
- Clinical observations by a medical doctor include progressive loss of function from nerve cells demonstrated through muscle weakness and sensory loss, enlarged nerves along the arm or neck, and the signs and symptoms listed above.
- A nerve conduction velocity (NCV) test can be performed, which is a test where nerves are electrically stimulated, and the speed of nerve impulses travelling is measured. Slow NCV, <38 m/s, indicates a possible diagnosis of CMT1 (with a normal range being >40-45 m/s).[4][2]
- A family history of inherited nerve disorders, muscle loss, or walking problems (that are otherwise undiagnosed) that follows an autosomal dominant pattern of inheritance can be an indicator of CMT1.
- Genetic testing that finds a disease-causing change in any of the genes listed above would confirm a diagnosis of CMT1, and can give more information on the specific subtype for the diagnosis. Genetic testing should be ordered by medical genetics professionals, such as a geneticist or a genetic counsellor[1].
Management of CMT1
There is currently no cure for CMT1. The management and treatment for CMT1 is symptomatic, and often involves a multidisciplinary team of healthcare professionals, including neurologists, physiatrists, orthopedic surgeons, and physical and occupational therapists.[6]
Treatment options include physical rehabilitation and exercise to improve muscle tone and mobility devices such as special shoes, ankle-foot orthoses, wheelchairs, and crutches[7][8][9]. Drug therapies are being studied and developed with the goal of neurological improvement in the future.[9][10]
Inheritance of CMT1
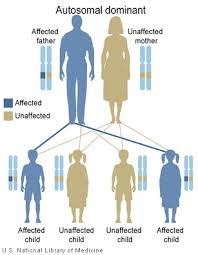
Each individual has two copies of every gene in their body: one from their mother and one from their father. CMT1 is considered a dominant disorder[4]. This means that only one copy of gene needs to have the disease-causing mutation for an individual to be affected by CMT1.
When an individual has children, they pass on one copy of each of their genes to their child, and there is an equal chance of passing on either copy. It is like a flip of a coin. This means that each child of an individual with CMT1 has a 50% chance of inheriting the gene copy that has a mutation. If a child inherits the mutation, they will also have CMT1.
Genetic Counselling for CMT1
Genetic counselling for CMT1 can be offered by qualified professionals, such as a geneticist or a genetic counsellor, if an individual is suspected to have the condition due to a personal or family history CMT1. During a genetic counselling appointment, the healthcare professional will collect the individuals personal and medical history to assess the risk that the individual has CMT1.
Genetic counsellors work alongside neurologists to help individuals understand how the disease may pass through their family, what their options are for genetic testing, and how to cope with a genetic condition in their family. Genetic counsellors are also trained to help individuals decide if genetic testing is right for them, as this is a very personal decision. Genetic counsellors are well equipped to provide individuals with resources or connect them with other care professionals or support groups if a diagnosis of CMT1 is obtained through genetic testing.
Genetic testing for an affected individual
Genetic counsellors can help arrange genetic testing to identify the specific gene causing the condition.[1] This type of testing can be offered to any affected individual, regardless of their age. Genetic tests require a blood or saliva sample from an affected individual. The test looks at the sequence of DNA from the sample to identify any specific disease-causing changes. Most commonly, single-gene testing for PMP22 happens first, since it accounts for most of the cases of CMT1 and about half the cases for CMT overall. If there are no disease-causing changes found in PMP22, a gene panel, which is a test that looks at multiple genes known to cause CMT1, may be used. If nothing is found in a gene panel, more extensive genetic tests such as whole exome sequencing (looking at all of the genes coding for proteins in an individual) or whole genome sequencing (looking at all of the DNA in an individual) may be used.
There are several possible results that can come up through genetic testing:
- A positive result means that a disease-causing change, or likely disease-causing change, was found in the genes looked at in the test. A positive result can give information about the underlying cause of the disease, and can be used to further categorize the diagnosis of CMT1 by its subtype.
- A negative result means that no disease-causing changes were found in the genes looked at in the test. For smaller tests, like single-gene testing for PMP22 or gene panels, a negative result just indicates that these specific genes did not have disease causing changes; however, this does not rule out the possibility that another gene contains a disease-causing change. A negative result can also happen in more extensive tests, such as whole exome or whole genome sequencing, which indicates that a specific genetic change could not be found to explain the condition.
- A variant of uncertain significance (VUS) result means that a change was found in the genes looked at, but it cannot be determined whether this change is disease-causing or just a normal change. Usually, this means that more research is needed to determine whether the gene change is disease-causing or not.
- Secondary findings can show up in more extensive tests, such as whole exome or whole genome sequencing. Since all of the genes of an individual are looked at in these tests, it is possible to find unexpected disease-causing variants in genes that are not related to CMT1, but are related to other conditions such as inherited cancer syndromes or types of heart diseases.
Predictive testing for asymptomatic individuals
Predictive genetic testing can be offered in a situation where a person's close relative, like a sibling or a parent, has been identified to carry a mutation that causes CMT1. In this case, even if that person has no symptoms, they can determine if they are at risk for developing CMT1.
It is not recommended to have genetic testing done for an minor (under 18 years old) who does not have any CMT1 symptoms, even if their close relative has a CMT1 diagnosis[1]. This recommendation is made as to maintain the autonomy of the child until they are old enough to decide for themselves if they would like to be tested. Predictive testing for minors is not offered due to the fact that there are no early interventions or treatments available for individuals to reduce the effects of having CMT1. This means that finding out earlier about a child's condition does not provide a way to prevent the disease, and having the information will not affect the severity of CMT1 symptoms in that child.
Psychosocial considerations
Genetic counsellors can also provide support to families surrounding the emotional and social impacts of having a CMT1 diagnosis. Receiving a diagnosis of CMT1 can result in a ripple effect in a person's life, such as altering family dynamics, and increased feelings of isolation. Concerns related to a diagnosis of CMT1 include, but are not limited to:
- Relief from receiving a final diagnosis
- Increased anxiety and depression
- feelings of isolation or being misunderstood by healthcare providers, family and friends
- Chronic pain from the condition itself and as a result of medical procedures
- Grief over a person's previous lifestyle due to lifestyle changes made to manage or cope with symptoms
- Stigmatization based on altered body image from feet malformations, using a wheelchair or altered walking patterns
- Fear of symptoms getting worse
- Distress from decreased employment or social engagement due to altered mobility
- Guilt of passing CMT1-causing gene to one's children, or worries that their future children may have CMT1
Patient Resources
For more information about CMT, visit:
- Hereditary Neuropathy Foundation of Canada
- Hereditary Neuropathy Foundation (USA)
- Charcot-Marie-Tooth Association (USA)
- National Organization for Rare Disorders (NORD)
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Bird TD. Charcot-Marie-Tooth (CMT) Hereditary Neuropathy Overview. 1998 Sep 28 [Updated 2022 Sept 29]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2022.
- ↑ 2.0 2.1 2.2 2.3 2.4 Ramchandren, S. (2017). Charcot-Marie-Tooth Disease and Other Genetic Polyneuropathies. CONTINUUM: Lifelong Learning in Neurology, 23(5), 1360–1377. doi: 10.1212/CON.0000000000000529.
- ↑ 3.0 3.1 Nagappa, M., Sharma, S., & Taly, A. B. (2022). Charcot Marie Tooth. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK562163/
- ↑ 4.0 4.1 4.2 4.3 4.4 Morena, J., Gupta, A., & Hoyle, J. C. (2019). Charcot-Marie-Tooth: From Molecules to Therapy. International journal of molecular sciences, 20(14), 3419. doi:10.3390/ijms20143419
- ↑ 5.0 5.1 Vallat, J.-M., Mathis, S., & Funalot, B. (2013). The various Charcot–Marie–Tooth diseases. Current Opinion in Neurology, 26(5), 473. https://doi.org/10.1097/WCO.0b013e328364c04b
- ↑ McCorquodale, D., Pucillo, E. M., & Johnson, N. E. (2016). Management of Charcot-Marie-Tooth disease: improving long-term care with a multidisciplinary approach. Journal of multidisciplinary healthcare, 9, 7–19. doi:10.2147/JMDH.S69979
- ↑ Kenis-Coskun, O., Matthews, D.J. (2016). Rehabilitation issues in Charcot-Marie-Tooth disease. J Pediatr Rehabil Med 9, 31–4.
- ↑ Miniou, P., & Fontes, M. (2021). Therapeutic Development in Charcot Marie Tooth Type 1 Disease. International Journal of Molecular Sciences, 22(13), Article 13. https://doi.org/10.3390/ijms22136755
- ↑ 9.0 9.1 Pisciotta, C., Saveri, P., & Pareyson, D. (2021). Updated review of therapeutic strategies for Charcot-Marie-Tooth disease and related neuropathies. Expert Review of Neurotherapeutics, 21(6), 701–713. https://doi.org/10.1080/14737175.2021.1935242
- ↑ Kiepura, A.J. and Kochanski, A. (2018). Charcot‑Marie‑Tooth type 1A drug therapies: role of adenylyl cyclase activity and G‑protein coupled receptors in disease pathomechanism. Acta Neurobiol Exp 78, 198-209. doi: 10.21307/ane-2018-018