Course:MEDG550/Student Activities/Achondroplasia
Achondroplasia is the most common form of dwarfism among humans.[1] The condition has been known for many centuries, as documented in ancient Egyptian, Greek, and Roman art.[2]A characteristic feature of achondroplasia is short stature caused by improper development of the bones in early life.[3] Achondroplasia is one of a group of genetic conditions caused by changes in the FGFR3 gene and occurs in approximately 1/10,000 to 1/30,000 live births.
What does achondroplasia look like?

Craniofacial Features
- Large head with prominent forehead
- Flattened nasal bridge
- Undergrowth of midface
- Narrow nasal passages
- Protrusion of lower jaw
- Dental crowding
Other Features[4]
- Short stature with normal trunk length
- Average adult male height: 131cm ± 5.6 (approx. 4 ft. 3 in.)
- Average adult female height: 124cm ± 5.9 (approx. 4 ft. 1 in.)
- Low muscle tone
- Shortening of upper arms and thighs
- Fluid in brain in infancy
- Joints moving beyond normal range of motion
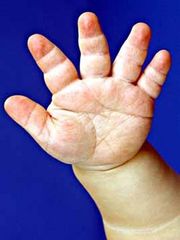
- Trident hands (see photo)
- Prominent buttocks
- Bowing of legs
- Protuberant abdomen
Complications
- Motor delay in infancy and early childhood
- Sleep disturbances
- Breathing disorders
- Lower body weakness/paralysis due to compression of spinal cord
- Poor head control
- Obesity
What causes achondroplasia?



Achondroplasia is caused by critical change in one copy of the FGFR3 gene that is located on chromosome 4.[5] FGFR3 is important in the process of converting cartilage to bone during early development. These changes in the gene cause FGFR3 to become overactive, which in turn disturbs normal bone formation.[6] The result is reduced bone size. FGFR3 is the only known gene known to be implicated in achondroplasia. All individuals who have the known change in the FGFR3 gene will have achondroplasia.
How is achondroplasia inherited?
Individuals with achondroplasia have a change in one copy of the FGFR3 gene causing it to have abnormal functioning. The second copy of the FGFR3 gene functions normally, meaning the individual is heterozygous for the change. The majority of the time (80% of cases) the mutation is new in the child of parents of average stature.[7] Men of average height who have children at a later age (>50 years) have a greater chance of having a child with achondroplasia.[8] It is thought that sperm that carry a FGFR3 gene with the achondroplasia-associated change have an advantage over sperm that carry an unchanged FGFR3 gene.[9]The chance of having another child with achondroplasia of parents of average stature is very low. Sometimes, a couple with average stature will have more than one child with achondroplasia. A possible explanation for this occurrence is gonadal mosaicism. This means that a subset of cells in either the mother's ovaries or in the father's testes that go on to make eggs or sperm, respectively, carry the FGFR3 change that causes achondroplasia.
In the remainder of cases (20%), the change is inherited from a parent with achondroplasia following an autosomal dominant pattern of inheritance. This means that only one copy of the FGFR3 gene with the change is necessary to cause achondroplasia.
A parent with average stature and a parent with achondroplasia
An individual who has achondroplasia has a 50% chance of passing the FGFR3 gene with the change to his/her child, and subsequently has a 50% chance of having a child with achondroplasia.
A couple who both have achondroplasia
Individuals with achondroplasia may have a partner who also has achondroplasia or a different form of inherited dwarfism. When both parents have achondroplasia, they have a 25% chance of having a child of average stature, a 50% chance of having a child with achondroplasia, and a 25% chance of having a child with homozygous achondroplasia (both copies of FGFR3 with the change are passed down). Homozygous achondroplasia is a lethal condition. Prenatal diagnosis by amniocentesis or chorionic villus sampling is available as an option for these couples.
A parent with achondroplasia and a parent with hypochondroplasia
If one parent has achondroplasia and the other parent has a different form of short stature, the chances of affected children differ. For instance, hypochondroplasia is an inherited form of short stature which is caused by a different mutation in the FGFR3 gene. A parent with achondroplasia and a parent with hypochondroplasia have a 25% chance of having a child of average stature, a 25% chance of having a child with achondroplasia, a 25% chance of having a child with hypochondroplasia, and a 25% chance of having a child who is a double heterozygote. A double heterozygote has inherited a copy of the gene with the change implicated in achondroplasia and another copy of the gene with the change implicated in hypochondroplasia. The clinical features of a double heterozygote are much more severe. Prenatal diagnosis is available for these couples.
How is achondroplasia diagnosed?
After Birth
Achondroplasia is primarily diagnosed based on features seen by X-ray. Key findings include narrowing of the spine, underdeveloped facial bones and skull base, and shortening of the upper limbs (upper arms and thighs).[10] Genetic testing by molecular analysis of the FGFR3 gene can be used to confirm an uncertain diagnosis.
During Pregnancy
Ultrasound
Achondroplasia can be identified prenatally after 26 weeks with a 3rd trimester ultrasound. It is often discovered incidentally. Ultrasound findings indicative of achondroplasia include shortened limbs, increased biparietal diameter (measurement of baby’s head from ear-to-ear), and a low nasal bridge.[11]
Prenatal Diagnostic Testing
Invasive diagnostic testing by amniocentesis or chorionic villus sampling is available for achondroplasia. Amniocentesis involves the collection of a small amount of fluid surrounding the baby and can be performed between 15 and 23 weeks of pregnancy. Chorionic villus sampling involves the collection of a small sample of placental tissue and can be performed between 11 and 14 weeks of pregnancy. Molecular testing can be completed on either the fluid or the placental tissue to look for the change in the FGFR3 gene. However, both procedures come with a risk of miscarriage. It is advised that couples wishing to undergo diagnostic procedures consult with a genetic counselor or physician.
How is achondroplasia treated/managed?
Considering the variability in symptom presentation, individuals with achondroplasia should be carefully evaluated and managed. Weight, height, and head circumference measurements should be monitored using growth curves specific for individuals with achondroplasia. Neurological examinations should be completed using magnetic resonance imaging (MRI), computed tomography (CT), somatosensory evoked potentials (SEP) test and sleep studies.[12] Ear infections are common due to abnormal bone formation in the middle ear. It is important that these middle ear infections are properly treated. Prevention and monitoring of obesity should begin in early childhood.
A controversial treatment available for children with achondroplasia is surgical limb lengthening. It involves breaking bones, usually the femur (thigh bone) and humerus (upper arm bone), and slowly stretching them during the healing process using orthopaedic appliances. It can add height of 15-30 cm.[13]
Even though growth hormone deficiency is not implicated in achondroplasia, studies have investigated the use of supplemental growth hormone as a possible treatment. Generally the outcomes have been poor; therefore, growth hormone is not currently recommended as a treatment for individuals with achondroplasia.[14]
Genetic Counselling
Genetic counselling is available for couples of average stature when achondroplasia is identified prenatally. Genetic counsellors provide couples with support and information regarding achondroplasia. Additionally, they provide couples with their options for prenatal diagnosis and help to facilitate decision making. Genetic counsellors will also meet with couples who both have achondroplasia or couples with one partner who has achondroplasia either before they become pregnant or during pregnancy. Genetic counsellors can help couples understand and adapt to the reproductive implications of achondroplasia.
How might a diagnosis of achondroplasia affect my family?
A diagnosis with achondroplasia can have emotional and social implications on individuals and families. It is important to note that every individual and family will live with and cope with achondroplasia differently. Seeking out a genetic counsellor may help individuals to cope with any emotions that they may be feeling that stem from their experience with achondroplasia.
Implications for affected individuals
Due to the chronic nature of achondroplasia, individuals affected with the condition can sometime have a lower quality of life compared to those without the condition. This is often explained by physical restrictions and limitations as well as other challenges in daily life that can start from childhood and persist into adulthood[15]. Some of these challenges can include medical problems, stigma, low self esteem and limited career options[16]. Many of these challenges can be improved with the use of services and supports available in the resources found below.
Individuals with achondroplasia have also noted many advantages to having the condition. They believe that having achondroplasia can increase one’s strength of character, improve friendships while also allowing for the chance to meet more people, and can make people more tolerant to diversity[16].
Implications for parents and families
Parents and families of those with achondroplasia can also face some difficulties due to the diagnosis of the condition in their relative. Broadly parents may feel an impact in their caretaking responsibilities, emotional well-being, family, and work[15][17]. Indeed, parents may find it difficult to manage their child’s medical care, to help their child with self-care, and to advocate for their child. Emotionally parents may experience increased anxiety and worry due to uncertainties in the future of their child, worry about their child’s health, safety, and social relationships. All of this may be extremely overwhelming for some parents. A diagnosis in a child may also cause familial strain while also requiring adaptation of family activities. Due to the possibility of increased medical appointments for their children many parents may also require time off of work[17].
Resources and Support
There are numerous groups across North American devoted to connecting and facilitating communication between people with achondroplasia and other forms of dwarfism. The different groups aim to provide support and information to individuals with dwarfism and their families.
Little People of British Columbia
Association of Little People of Alberta
Association québécoise des personnes de petite taille
References
- ↑ Baujat, G., Legeai-Mallet, L., Finidori, G., Cormier-Daire, V., & Le Merrer, M. (2008). Achondroplasia. Best Pratice & Research Clinical Rheumatology, 22(1), 3-18. doi:10.1016/j.berh.2007.12.008
- ↑ Horton, W.A., Hall, J.G., & Hecht, J.T. (2007). Achondroplasia. Lancet, 370, 162-172.
- ↑ Carter, E.M., Davis, J.G., & Raggio, C.L. (2007). Advances in understanding etiology of achondroplasia and review of management. Current Opinion in Pediatrics, 19(1), 32-37. doi: 10.1097/MOP.0b013e328013e3d9
- ↑ Chen, H. (2012). Achondroplasia. Atlas of Genetic Diagnosis and Counseling, 21-34. DOI 10.1007/978-1-4614-1037-9_3
- ↑ Chen, H. (2012). Achondroplasia. Atlas of Genetic Diagnosis and Counseling, 21-34. DOI 10.1007/978-1-4614-1037-9_3
- ↑ Richette, P., Bardin, T., & Stheneur, C. (2008). Achondroplasia: From genotype to phenotype. Joint Bone Spine, 75, 125-130. doi:10.1016/j.jbspin.2007.06.007
- ↑ Bober, M. & Duker, A. (2013). Achondroplasia. Orphanet. Retrieved from http://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=GB&Expert=15
- ↑ Kovac, J.R., Addai, J., Smith, R.P., Coward, R.M., Lamb, D.J., Lipshultz, L.I. (2013). The effects of advanced paternal age on fertility. Asian Journal of Andrology, 15, 723-728
- ↑ Horton, W.A., Hall, J.G., & Hecht, J.T. (2007). Achondroplasia. Lancet, 370, 162-172.
- ↑ Chen, H. (2012). Achondroplasia. Atlas of Genetic Diagnosis and Counseling, 21-34. DOI 10.1007/978-1-4614-1037-9_3
- ↑ Baujat, G., Legeai-Mallet, L., Finidori, G., Cormier-Daire, V., & Le Merrer, M. (2008). Achondroplasia. Best Practice & Research Clinical Rheumatology, 22(1), 3-18. doi:10.1016/j.berh.2007.12.008
- ↑ Carter, E.M., Davis, J.G., & Raggio, C.L. (2007). Advances in understanding etiology of achondroplasia and review of management. Current Opinion in Pediatrics, 19(1), 32-37. doi: 10.1097/MOP.0b013e328013e3d9
- ↑ Horton, W.A., Hall, J.G., & Hecht, J.T. (2007). Achondroplasia. Lancet, 370, 162-172.
- ↑ Horton, W.A., Hall, J.G., & Hecht, J.T. (2007). Achondroplasia. Lancet, 370, 162-172.
- ↑ 15.0 15.1 Witt, S., Kolb, B., Bloemeke, J., Mohnike, K., Bullinger, M., & Quitmann, J. (2019). Quality of life of children with achondroplasia and their parents - a German cross-sectional study. Orphanet Journal of Rare Diseases, 14(1), 194. doi:10.1186/s13023-019-1171-9
- ↑ 16.0 16.1 Gollust, S. E., Thompson, R. E., Gooding, H. C., & Biesecker, B. B. (2003). Living with achondroplasia in an average-sized world: An assessment of quality of life. American Journal of Medical Genetics Part A, 120A(4), 447-458. doi:https://doi.org/10.1002/ajmg.a.20127
- ↑ 17.0 17.1 Pfeiffer, K. M., Brod, M., Smith, A., Gianettoni, J., Viuff, D., Ota, S., & Charlton, R. W. (2021). Assessing the impacts of having a child with achondroplasia on parent well-being. Quality of Life Research, 30(1), 203-215. doi:10.1007/s11136-020-02594-3