Course:FNH200/Lessons/Lesson 12/Page 12.5
12.5 Examples of Natural Constituents as Toxicants
Glycoalkaloid-Cholinesterase Inhibitors
- The name "cholinesterase inhibitors" refers to a variety of chemicals which are able to inhibit the activity of the enzyme cholinesterase. This enzyme is found in nerve tissues and plays an important role in the transmission of nerve impulses. When its function is inhibited, nerve function is affected.
- Solanine is an example of such inhibitors. Solanine can is found in potatoes, normally at levels of 2-13 mg/100 g fresh weight,
- the bulk of market potatoes probably contain only 3-6 mg/100 g.
- Levels as high as 80-100 mg/100 g, have been reported particularly if the potatoes have undergone greening (reaction of potato tubers on exposure to sunlight).
- Solanine is insoluble in water and is not lost or destroyed when potatoes are cooked. Because it is found primarily in the skin of potatoes, the peeling of potatoes reduces the concentration markedly.
Clinical symptoms of solanine poisoning: gastrointestinal disturbances and certain neurological disorders. Solanine poisoning can result in death. However, ingested solanine is less likely to cause toxic symptoms than an injected dose because it is not readily absorbed and is fairly rapidly excreted by way of the feces and the urine. Humans display drowsiness, increased sensory sensitivity, and difficulty in breathing after an oral dose of 100 mg (approximately 2-8 mg/kg body weight). Higher doses may cause vomiting and diarrhea.
| Want to learn more? |
|
- Although the chemical solanine itself is quite a potent toxicant, the hazard of solanine poisoning by consumption of potatoes is quite low.
- Potato growing and handling practices minimize the opportunity for greening, and hence minimize the production of abnormally high levels of solanine.
- Seed potatoes which are genetically low in solanine content are used, and during the growing season the potato plants are hilled, thereby decreasing exposure of the potato to light.
- After harvesting, potatoes are put into storage facilities which minimize exposure to light.
- Consumers who ignore the hazards of consuming potatoes that have turned green during improper storage at home would face a high risk of poisoning by solanine!
| Want to learn more? |
|
Cyanogenic Glycosides
- Cyanogenic glycosides yield hydrogen cyanide (HCN) upon treatment with acid or particular hydrolytic enzymes.
- They are found widely in higher plants, and also occur in ferns, moths and insects. Among the plants used as food which contain one or more of these toxicants are: cassava, sweet potato, yam, maize, bamboo, sugar cane, peas, lima beans, almonds, lime, apple, pear, cherry, apricot and plum.
- Cyanide is very rapidly absorbed from the gastrointestinal tract and produces recognizable symptoms at both fatal and non-fatal levels.
With fatal doses of HCN, death results from the general anoxic condition caused by the inhibition of cytochrome oxidase, with which the HCN complexes. Cytochrome oxidase is an important component of the oxidative phosphorylation cycle which occurs in the mitochondria of cells. Inhibition of the enzyme system causes the death of the cells.
Since the HCN binds reversibly to the cytochrome oxidase, non-fatal doses permit recovery by means of respiratory exchange and metabolic detoxification processes.
For humans, the minimum lethal dose of HCN taken orally has been estimated to be between 0.5-3.5 mg/kg of body weight. It is obvious from the data in Table 12.3 that consumption of 100 g of bitter almonds by a 70 kg man could produce a dose of 3.57 mg/kg of body weight, a fatal dose even for the least sensitive individual.
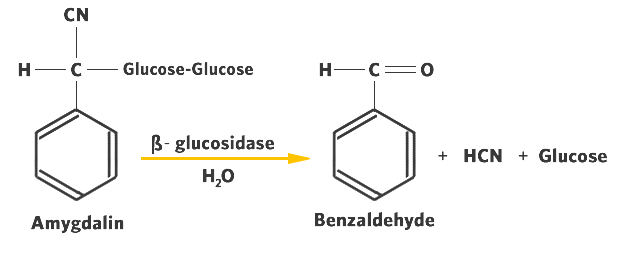
- Amygdalin is an example of a cyanogenic glycoside. It is found in bitter almonds and a number of fruit pits.
- Amygdalin, having a carbohydrate component ("glycoside") and hydrogen cyanide is hydrolyzed to form HCN, glucose and benzaldehyde ( Figure 12.2).
The enzymes responsible for the hydrolysis of cyanogenic glycosides are generically called ß-glycosidases. They are highly specific for the ß-glycosidic linkage that is characteristic of the cyanogenic glycosides.
These plant enzymes differ from the a-glycosidases (amylases) of the mammalian digestive tract that hydrolyze only a-glycosidic bonds such as those found in starch.
- Hydrolysis of cyanogenic glycosides can occur during the cutting, crushing, bruising or maceration of tissue prior to consumption, or can be initiated upon the maceration of the tissue during eating.
- Cyanide poisoning can therefore occur when enough of a plant material that is rich in cyanogenic glycosides and enzymes is consumed, or when such a material has been prepared with insufficient care to remove the HCN accumulated during preparation.
The scientific literature records numerous incidents of accidental poisonings by bitter almonds, cassava, and lima beans. Table 12.3 gives some indication of the amount of cyanide that may be produced from various plant foodstuffs.
Table 12.3 Data showing the potential yield of cyanide in some plant foodstuffs. Adapted from: Liener, I.E. (ed.). 1980. Toxic constituents of plant foodstuffs. New York: Academic Press.
| Plant | HCN Yield
(mg/100 g material) |
|---|---|
| Bitter almonds | 250 |
| Bitter cassava root | 53 |
| Bitter cassava root cortex (dried) | 245 |
| Lima bean, American white | 10 |
| Lima bean, Java coloured | 312 |
- In parts of the world where some of these cyanide producing plants are used extensively for food, means of preparation have been developed to remove or hydrolyze the glycosides and to destroy the ß-glucosidase that is present. While these steps minimize the hazard, they do not completely eliminate it.
It is important, however, that while the cyanide-producing capacity of a plant is important in determining its toxicity, there are other factors which must also be considered. These factors include the size and kind of subject, the speed of ingestion, the type of food ingested simultaneously with the cyanogen, the presence of active degradative enzymes both in the plant and in the subject's digestive tract, and the subject's ability to detoxify the HCN.
The hazard associated with the presence of cyanogenic glycosides in food for those individuals who consume large quantities of the affected plant foodstuff, is much more significant than those who accidentally eat the occasional apple seed or apricot kernel.
Other Natural Toxicants
There are a number of other natural toxicants listed in Table 12.1 for which only a very brief comment will be made in this course.
Protease inhibitors
- Proteinaceous compounds found in many of the legume species.
- have the ability to complex to, and thereby interfere with, certain proteolytic enzymes.
- Proteins need to be hydrolyzed into their constituent amino acids by digestive enzymes such as trypsin and chymotrypsin. If these enzymes are rendered inactive by complexing inhibitors, the body cannot fully hydrolyze the proteins, thereby creating the possibility of amino acid deficiencies.
Nitrates
- Widely found constituents of plant materials, especially green leafy plants.
- Nitrates themselves are not very toxic; however, bacteria can reduce them to nitrites.
- A primary concern about nitrites is their ability to interact chemically with hemoglobin, interfering with the blood's ability to transport the required oxygen to the body's cells. By a rather complex series of reactions, not only bacteria but also metabolic pathways within the digestive system of humans can utilize nitrate/nitrite as a precursor for the formation of nitrosamines, potent carcinogens.
Allergens
- Have the ability to induce allergic reactions in sensitive individuals. Foods that are most frequently reported to cause allergic responses are: cereals such as wheat, rye and rice; legumes such as peas, peanuts and soybeans; tree nuts; milk; eggs; and seafoods such as shrimp, crab and lobster.
- The case of allergens provides a good example of the need to consider individual (genetic) differences in assessing risk and hazards. For most of us, consumption of the foods listed above does not pose any significant risk. However, for someone who is severely allergic to a particular food or food component, accidental ingestion may lead to a life-or-death situation!