Course:FNH200/2012w Team25 Sweeteners
Introduction
Stevia and its History
Stevia is a perennial shrub that is native in Paraguay but is also cultivated in Asia, Europe and Canada [1]. It contains compounds called steviol glycosides within its leaves which produces an intensely sweet flavour that makes it a good natural substitute for sucrose. It has been used by native cultures as a natual sweetener to counteract the bitter tastes in plant- based drinks and medicine. According to Health Canada, steviol glycosides are non-carcinogenic and are non- caloric compounds that have been deemed safe for consumption. The leaves of Stevia can be used fresh, dried or dried and powdered. [2]. Some micro and macromolecules that can be found in Stevia are calcium, potassium, iron, protein, and ash of crude fibre. [3] The increase in popularity of this product has began raising questions such as: How is stevia transformed into a white powder or its other forms? How is it regulated in Canada and other nations? As well as how is it digested and metabolized within our bodies?

Taste
How, you may ask, could a leaf produce such a sweet taste?
Steviol glycosides, namely, rebaudioside A-F, steviolbioside, and dulcoside A, are responsible for the sweet taste of Stevia that is 200 to 300 times sweeter than sucrose. There is a delay in its production of the sweet taste on the taste buds. However, the sweet sensation lasts longer than sucrose and can enhance the flavours of different foods [4].
Steviol Glycosides: The sweet components of Stevia
| Stevial Glycosides | Molecular Weight | Multiply steviol glycoside concentration by factor to obtain steviol equivalents |
|---|---|---|
| Stevioside | 804.88 | 0.395 |
| Rebaudioside A | 967.03 | 0.329 |
| Rebaudioside C | 951.02 | 0.334 |
| Dulcoside A | 788.88 | 0.403 |
| Rubusoside | 642.73 | 0.496 |
| Steviolbioside | 642.73 | 0.496 |
| Rebaudioside B | 804.88 | 0.395 |
| Rebaudioside D | 1129.15 | 0.282 |
| Rebaudioside F | 936.99 | 0.339 |
Physical Properties
It is soluble in water and the dried powder form degrades at 200 degrees Celsius of different foods and can therefore, be used in baking. These compounds are most stable at pH 2 to 10 at 80 degrees Celsius[5].
Physical Appearance
Crude Product: Green crushed leaves
Pure Product: White powder or concentrated liquid
-
Crude Product
-
Concentrated Powder
Regulations
How is Stevia Regulated?
Stevia is regulated by Health Canada, which determines how stevia is to be used, in what amount, and in what form. Even though stevia has been used in some countries, it wasn’t until November 30, 2012 when Health Canada included steviol glycosides to the list of permitted sweeteners in Canada. Health Canada also includes a list of permitted foods it can be used in and their amounts.
Foods and Labels of Steviol Glycoside

Steviol glycosides are allowed in breakfast cereals, beverage mixes, fillings, yogurt, breath freshener products, chewing gum, and many other products which are included in a list on the Health Canada website. For each of these food products, Health Canada has indicated the percentage of the product permitted to be steviol glycosides. When used as a tabletop sweetener or as a flavour enhancer, the amount used must be considered as “Good Manufacturing Practice”, meaning that only the minimum amount needed for the desired outcome is used. This is the pure stevia extract that is allowed as a sweetener but there is also a leaf form and crude extract form. These other forms are less than the 95 percent pure extract form and therefore are not considered a food additive or sweetener. Due to the fact that Health Canada is not able to provide full evidence of the safety of this crude form, stevia leaves and the crude extract form are considered a food ingredient. This is because there is no specific criteria for a food ingredient so they can avoid having to be assessed and approved which is a mandatory requirement for additives. The leaves and crude product are only permitted for personal cooking and cannot be used in manufactured food products. The people who sell the impure product are legally responsible for it.[6]
Canadian Regulations for the Additive: Steviol Glycosides
| Permitted in or on | Maximum Level of Use |
|---|---|
| Table-top Sweeteners | Good Manufacturing Practice |
| Breakfast cereals | 0.035% (calculated as steviol equivalents) |
| Unstandardized beverages mixes; Unstandardized beverages; Unstandardized beverage concentrates | 0.02% (calculated as steviol equivalents) in beverages as consumed |
| Unstandardized dessert mixes; Fillings; Filling mixes; Toppings; Topping mixes; Unstandardized desserts; Yogurt | 0.035% (calculated as steviol equivalents) in products as consumed |
| Breath freshener products; Chewing gum; | 0.35% (calculated as steviol equivalents) |
| Unstandardized fruit spreads; Unstandardized purées and sauces; Unstandardized table syrups | 0.035% (calculated as steviol equivalents) |
| Peanut spreads; Nut spreads; Unstandardized salad dressings | 0.035% (calculated as steviol equivalents) |
| Unstandardized condiments | 0.013% (calculated as steviol equivalents) |
| Confectionery glazes for snack foods; Sweetened seasonings or coating mixes for snack foods | 0.035% (calculated as steviol equivalents) |
| Unstandardized confectionery (except unstandardized chocolate confectionery); Unstandardized confectionery coatings (except unstandardized chocolate confectionery coatings) | 0.07% (calculated as steviol equivalents) |
| Unstandardized chocolate confectionery ; Unstandardized chocolate confectionery coatings | 0.035% (calculated as steviol equivalents) |
| Unstandardized bakery products; Baking mixes | 0.035% (calculated as steviol equivalents) in products as consumed |
| Note: The units for the maximum level of use are expressed on the basis of steviol equivalents rather than steviol glycosides, which is consistent with the approach used internationally. |
How much stevia is safe for you?
For the pure form, which is considered an additive, there is an average daily intake (ADI) of 4 milligrams of steviol glycoside per kilogram body weight. If you consider a person that weighs 50 kilograms, they are allowed to consume 200 milligrams of steviol glycoside. Only about one packet of stevia is needed to sweeten a cup of coffee or tea and only contains about 50 milligrams of steviol glycoside. This means that you can can have about 4 packets of stevia before reaching the ADI. Also, this ADI is one hundred times higher than the maximum dose tested on an animal before it starts to have negative effects. This ADI is without cautionary labelling and additional safety evidence. If a product exceeds this ADI, it must be labelled “Consult a healthcare practitioner prior to use if you have high blood pressure”. The crude product is approved by Health Canada to be a medical ingredient in some natural healthcare products such as vitamins and supplements, but again, the seller has complete legal responsibility for the product they are selling.[8]
US Regulations
Stevia, like many other products, was legalized in the United States well before it was legalized in Canada. In December of 2008, the FDA approved stevia as a zero calorie sweetener. It is the pure extract of stevia that is approved which must be greater than 90 percent of the pure concentrated form. The FDA granted this form of stevia as “Generally Recognized as Safe” (GRAS), which is defined as being a chemical or substance added to food which is considered safe by qualified scientists and other experts, but is not officially approved as food additive due to the lack of scientific research.
Would You Choose To Consume Stevia?
Now that it is available in Canada and the United States, it is up to each consumer to decide for themselves if they want to consume this product.
Processing
Processing stevia can be done at home or in large-scale commercial processing plants.
At Home
At home, the stevia leaves can be dried on a screen in the sun or in a tray drier until the leaves become crisp and waterless. The dried leaves are most desirable when the green colour is retained. The leaves can then be grinded and crushed mechanically or by hand until it becomes a finely milled green powder. The fine powder is what we know as stevia.[9]
Commercial Processing
In commercially produced stevia, steviol glycosides, the sweet compounds in stevia, are extracted from the leaves through a process of reverse osmosis.
1.Drying
First, the stevia leaves are dried.
2.Reverse Osmosis
Once dried, the leaves are placed into tall pressurized glass columns with water, ethanol, or methanol as the solvent. The extraction procedure differentiates depending on temperature; with the extract having more impurities and colour pigment in higher temperature and lower pH. The steviol glycosides will diffuse out of the leaves in exchange with the solvents entering the leaves. The temperature for extraction can range from 0-35 degrees C.
3.Purification (Diafiltration)
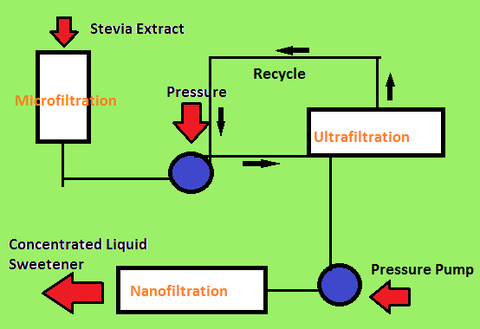
After the extraction of steviol glycosides, the next step is purification. Purification of the extract requires membrane-based filtrations known as diafiltration. The temperatures for diafiltration can range as high as 80 degrees C. [10]
Microfiltration, ultrafiltration, and nanofiltration are different levels of diafiltration. Each level of filtration has different permeability in the membranes and removes different sizes of impure particles.
- -Microfiltration
- Microfiltration removes larger suspended impure particles along with some steviol glycoside particles. However, the lost steviol glycoside particles can be recovered through further microfiltration of the permeated solution.
- -Ultrafiltration
- Once treated with microfiltration, the solution is ultrafiltrated where finer impure particles are removed.
- -Nanofiltration
- The final membrane-based filtration is nanofiltration. Nanofiltration at 80 degrees C removes most non-steviol glycoside particles and the result is a concentrated liquid sweetener.
4.Spray Dry (Optional)
Further processing can involve spray drying [11]. In spray drying, the liquid permeate is sprayed into high velocity warm air. The warm air quickly and evenly dehydrates the liquid and the result is a concentrated, white, powdered sweetener [12]
Packaging
-
Liquid Form
-
Powder Form
Digestion
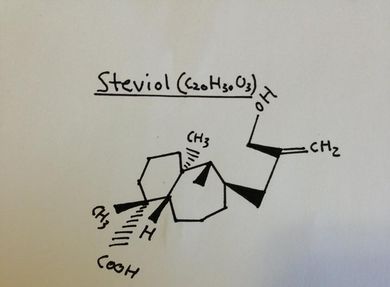
One might wonder if stevia can cause any discomfort or side effects due to humans being unable to fully metabolize and digest stevia and it has been well documented that some individuals have experienced side effects such as dizziness, nausea, upset stomach, numbness, and more. However, stevia should have no effect on the stomach or cause no gastrointestinal disturbances because after stevia is broken down to steviol, steviol is no longer altered by human metabolism. The majority of the steviol is “absorbed and glucuronidated (a bond intended to help them clear out of the blood) in the liver”. These newly bonded glucuronides are then released into the blood, filtered by the kidneys into urine and excreted. Therefore, Stevia should have no effect on your body except for the sweet taste it imparts. Stevia leaves contain the diterpene glycoside molecules stevioside, steviolbioside, rebaudiosides A-F and dulcoside A. These molecules are responsible for the sweet taste of stevia. Stevioside and rebaudioside A are the most common and abundant glycosides in stevia, with dulcoside A being almost negligible. Aside from just being a sweetener, stevia extracts have also been shown to have many beneficial health effects on humans; some of which include being antioxidants, antihyperglycemic, noncariogenic, and anti-human ratavirus activities Upon entering the mouth and imparting a sweet sensation to the taste buds, stevioside molecules in the sweetener pass through the entire human digestive tract without being completely absorbed or broken down. Thus, the sweetener yields 0 calories and doesn’t contribute to high blood sugar levels which is essential for individuals suffering from diabetes or are struggling to lose weight.
The metabolism of stevia has been studied in both animals and humans. The most popular animals for experimentation are rats and hamsters due to their similar GI tracts in comparison with humans. In one study, hamsters were fed radiolabeled stevioside, and it was later discovered that the digestive enzymes α-amylase, pancreatin and pepsin were unable to hydrolyze stevioside and rebaudioside A. However, in rats, the intestinal microflora were able to degrade the sweeteners to their aglycon steviol. This meant that only studying hamsters or rats led to inconclusive results in terms of the metabolism of stevia, and human subjects were needed.


Due to previously limited research, The Department of Food Science and Microbiology of the University of Milan conducted an experiment to gain further insight into the effects of human microflora with the steviol glycosides stevioside and rebaudioside A. As quoted from their report, “the aim of this study was to investigate the in vivo transformation of stevioside and rebaudioside A after incubation with human microflora, the influence of these sweeteners on human microbial fecal community and which specific groups metabolize preferentially stevioside and rebaudioside A.” The experiment composed of collecting the feces of 11 healthy individuals (six men and five women) of ages 20-50. Feces of five subjects were tested with glucose or stevioside, feces of the six others were tested with rebaudioside A or glucose, all under anaerobic conditions[13].
According to Dr. Fishberg MD, PHD, Scientific Advisory Board Member, Global Stevia Institiute, Reb A is first metabolized by the microbes located in our colon to stevioside, at the same time releasing a glucose molecule as it is converted to steviol. Although the released glucose molecule is not absorbed, it is used by the bacteria in the colon for energy. This correlates with the results from the experiment conducted by the University of Milan where the stevioside molecules were initially hydrolyzed to steviolbioside, but this intermediate was then quickly metabolized to steviol
The results from the University of Milan experiment show that stevioside was degraded to its aglycon steviol after approximately 10 hours of incubation with human microflora. Rebaudioside A was also metabolized to steviol by human microflora, but over a longer period of time (72 hours). However, human microflora did not hydrolyze steviol any further. The results of the two studies are in agreement with Dr. Fisberg, MD, PHD, Scientific Advisory Board Member, Global Stevia Institiute, who has once claimed “it has been demonstrated that the conversion rate of reb A and stevioside are similar between rats and humans, with the conversion from stevioside to steviol more rapid than that of reb A to steviol in both species”[14]
Stevia Vs. Sucrose
When comparing the digestion and absorption of Stevia and sucrose, it is clear that Stevia is more beneficial to the human body’s metabolism. Stevia, composed of stevioside, steviolbioside, dulcoside A and rebaudiosides A to F, yields an energy density of zero kilocalories per gram [15]. Since Stevia has an energy density of 0 kcal/g, it will not cause any harm to those with hyperglycemia or diabetes. Studies show that the amount of Stevia decreases by 90 percent after approximately 96 hours of ingestion. In a study conducted with rats, stevia is found to be absorbed through intestinal cells in the caecum. However, the human digestion of stevia is not as well known so further research is needed.[16]
-
Sucrose - Table Sugar
-
Stevia
Benefits of Stevia Vs. Sucrose
| Properties | Stevia | Sucrose |
|---|---|---|
| Energy Density | 0 kCal/g | 3.94 kCal/g |
| Digestion | Starts in the intestine | Starts in the mouth |
| Components | stevioside, steviolbioside, dulcoside A and rebaudiosides A to F | glucose and fructose |
Sucrose is composed of the monosaccharaides glucose and fructose [17]. It has a yield of 3.94 kilocalories per gram [18]. The enzyme sucrase breaks down sucrose in the jejunum, but stevioside is not affected by the sucrase. However, a study suggests that the stevioside stimulates sucrase activity but there is insufficient research to scientifically explain why this phenomena happens [19]. The body hydrolizes sucrose into its monosaccharide components and absorbs glucose in the small intestine [20]. Glucose, however, has been linked to high blood pressure [21]. The remaining monosaccharide, fructose, has been hypothesized to lead to obesity and LDL cholesterol accumulation [22] . Since the hydrolyzing rate of the sucrose itself is faster than that of the glucose, it is possible to have more remnant glucose traces in the body [23]. Hyperglycemia is caused by the lack of insulin activity to regulate blood glucose level and may lead to type 2 diabetes, obesity or high blood pressure. Stevia does not cause any of these symptoms because it is not absorbed [24].
Team 25 Video: How to Make Stevia
Would you make your own stevia sweetener at home?
Future Research
Numerous researchers have found stevia to be a a safe product that is good weight loss product [25]. However, there is a lack of intensive research on the side effects of this product. It has been recommended that pregnant women not consume this product because of the lack of information regarding effects on pregnancies. There has also been some research on allergic reactions to stevia for people allergic to Asteraceae/Compositae family [26]. Some researchers also say that stevia can lower blood pressure sugar levels but other researchers dispute this claim [27]. It is essential for these areas or research to be more focused on since most of the time, researchers see and share the positive effects of products with consumers and later on determine that there are plenty of dangerous side effects, only after a percentage of the population have already consumed the product. In order to determine these side effects, researchers should keep track of Stevia consumption in the population and determine whether there are deleterious effects on consumers throughout a certain period of time. This will allow them to determine what effects Stevia has in both the long and the short term. Over the course of a few years, researchers can collect data from a sample of the population where one group consumes stevia at least three times a week while the other group would have no stevia consumption at all. Based on their stevia consumption, researchers can investigate whether or not there were significant differences between the control group (no stevia consumption group) and the group that consumed stevia. The results would then, be used to determine whether stevia can be correlated with harmful side effects or not.
Conclusion
After researching and analyzing many aspects of Stevia, we have determined that Stevia is a good alternative for sucrose. Stevia is regulated closely by Health Canada and has to meet strict guidelines regarding purity, amounts used, and the foods that it is allowed in. This ensures that the product meets the standards of professionals who have studied ,tested it and determined when it can be called “safe”. Stevia can be made at home but it is not the purest form so there is no guarantee that it is safe to consume and is only considered a food ingredient, not an additive. The product that is considered an additive and is allowed to be incorporated in to manufactured food products has been processed in a lab and is no less than 95 percent pure. This seems to be safe because of the high standards of processing that must be met[28]. Once inside the body, Stevia is not completely broken down and therefore, contributes zero calories to net energy intake[29]. According to many studies, it could be a good substitute for sugar for those with diabetes ,hypoglycemia, obesity, or high blood pressure[30]. It is also commonly used in certain countries around the world. From the information that we have found, we believe that stevia seems like a generally safe alternative to sucrose. However, there is still some discrepancy to whether or not it will cause long term negative effects. There are still more studies and research that can be done on stevia to determine how safe it is. Keeping that in mind, it is always a good idea to consume a generally new product like stevia in moderation.
Question
1. Question: What physical properties and processing aspects cause it to be difficult for microbial growth of the stevia product?
- Answer: Drying the leaves commercially to a high temperature kills microorganisms; because it is a dehydrated, the water activity is very low which makes it an undesirable environment for microorganisms to grow; the impermeable plastic or glass bottle packaging prevents microorganism growth by preventing moisture from contacting the product.
2. Question: Why is stevia a desirable sweetener substitute?
- Answer: Stevia is natural, non-caloric, and is beneficial for those suffering from phenylketonuria (PKU) because unlike aspartame, it does not contain phenylalanine. Also, stevia does not affect hyperglycemia or diabetes.
References
- ↑ Goyal, S.K. (2010/02/01). Stevia(Stevia reubaudiana) a bio-sweetener: a review. International journal of food sciences and nutrition, 1(61), 1-10.
- ↑ Sugar Substitutes. (2010, March 25). Health Canada. Retrieved March 5, 2013, from http://www.hc-sc.gc.ca/fn-an/securit/addit/sweeten-edulcor/index-eng.php
- ↑ Akora, E. (2010, December 31). Stevia: A Promising Herbal Sweetener. JK Science: Journal of Medical Education and Research, 12(4), 212.
- ↑ Sugar Substitutes. (2010, March 25). Health Canada. Retrieved March 5, 2013, from http://www.hc-sc.gc.ca/fn-an/securit/addit/sweeten-edulcor/index-eng.php
- ↑ Kroyer, G. (2010, May). Stevioside and Stevia-Sweetener in Food: Application, Stability, and Interaction with Food Ingredients. Journal fur Verbraucherschutz und Lebensmittelsicherheit, 5(2), 225-229.
- ↑ List of Permitted Sweeteners. (2012, December 12). Health Canada. Retrieved March 5, 2013, from http://www.hc-sc.gc.ca/fn-an/securit/addit/list/9-sweetener-edulcorant-eng.php
- ↑ List of Permitted Sweeteners. (2012, December 12). Health Canada. Retrieved March 5, 2013, from http://www.hc-sc.gc.ca/fn-an/securit/addit/list/9-sweetener-edulcorant-eng.php
- ↑ Sugar Substitutes. (2010, March 25). Health Canada. Retrieved March 5, 2013, from http://www.hc-sc.gc.ca/fn-an/securit/addit/sweeten-edulcor/index-eng.php
- ↑ Singh, S.D. and Rao, G.P. (2005/03/01). Stevia: The herbal sugar of the 21st century. Sugar tech: an international journal of sugar crops and related industries, 1(7), 17-24.
- ↑ Membrane-based separation scheme for processing sweeteners from stevia leaves. (2000, August). Food Research International. Retrieved March 16, 2013, from http://www.sciencedirect.com.ezproxy.library.ubc.ca/science/article/pii/S0963996900000983
- ↑ Application of tailor-made membranes in a multi-stage process for the purification of sweeteners from Stevia rebaudiana . (2011, April). Journal of Food Engineering. Retrieved March 16, 2013, from http://www.sciencedirect.com.ezproxy.library.ubc.ca/science/article/pii/S0260877410005248
- ↑ Course:FNH200/Lesson 08 . (2012, March 4). UBC Wiki. Retrieved March 16, 2013, from http://wiki.ubc.ca/Course:FNH200/Lesson_08
- ↑ Gardana C. , Simonetti P. , Canzi E., Zanchi R., Pietta P. (2003). Metabolism of Stevioside and Rebaudioside A from Stevia rebaudiana Extracts by Human Microflora. Journal of Agricultural and Food Chemistry, 51, 6618-6622.
- ↑ Global Stevia Institute. (2013). Metabolism of the Zero-Calorie Sweetener Stevia. Retrieved from: http://www.globalsteviainstitute.com/en/Default/ResourceLibrary/Articles/MetabolismoftheZeroCalorieSweetenerStevia.aspx
- ↑ Sugar Substitutes. (2010, March 25). Health Canada. Retrieved March 5, 2013, from http://www.hc-sc.gc.ca/fn-an/securit/addit/sweeten-edulcor/index-eng.php
- ↑ Koyama, E., Kitazowa, K., Ohori, Y., Izawa, O., Kakegawa, K., Fujino, A., Ui, M. (2003, March). In Vitro Metabolism of the Glycoside Sweeteners, Stevia Mixture and Enzymatically Modified Stevia in Human Internal Microflora. Food and Chemical Toxicity, 41(3), 359-374.
- ↑ Gray, G.M. and Ingelfinger, F.J. (1966, March). Intestinal absorption of sucrose in man: interrelation of hydrolysis and monosaccharide product absorption. The Journal of Clinical Investigation, 45(3), 388-398
- ↑ Donato, K. and Hegsted, D.M. (1985, August). Efficiency of utilization of various sources of energy for growth. Proceedings of the National Academy of Sciences of the United States of America, 82(15), 4866-4870
- ↑ Toskulkao, C. and Sutheerawattanaon, M. (1994, November). Effects of stevioside, a natural sweetener, on intestinal glucose absorption in hamsters. Nutrition Research, 14(11), 1711-1720
- ↑ Gray, G.M. and Ingelfinger, F.J. (1966, March). Intestinal absorption of sucrose in man: interrelation of hydrolysis and monosaccharide product absorption. The Journal of Clinical Investigation, 45(3), 388-398
- ↑ Giugilano, D., Ceriello, A., and Esposito, K. (2008, January). Glucose metabolism and hyperglycemia. The American Journal of Cllinical Nutrition, 87(1), 2175-2225
- ↑ Elliott, S.S., Keim, N.L., Stern, J.S., Teff, K. and Havel, P.J. (2002, November). Fructose, weight gain, and the insulin resistance syndrome. The American Journal of Clinical Nutrition, 76(5), 911-922
- ↑ Gray, G.M. and Ingelfinger, F.J. (1966, March). Intestinal absorption of sucrose in man: interrelation of hydrolysis and monosaccharide product absorption. The Journal of Clinical Investigation, 45(3), 388-398
- ↑ Meddings, J. (1997, May 1).Sucrose--how sweet is it?. Journal of pediatric gastroenterology and nutrition, 24(5), 621-622
- ↑ (2009). “Stevia”. WebMD. 23 March 2013. Retrieved from http://www.webmd.com/vitamins-supplements/ingredientmono-682-stevia.aspx?activeIngredientId=682&activeIngredientName=stevia&source=1.
- ↑ (2009). “Stevia”. WebMD. 23 March 2013. Retrieved from http://www.webmd.com/vitamins-supplements/ingredientmono-682-stevia.aspx?activeIngredientId=682&activeIngredientName=stevia&source=1.
- ↑ (2009). “Stevia”. WebMD. 23 March 2013. Retrieved from http://www.webmd.com/vitamins-supplements/ingredientmono-682-stevia.aspx?activeIngredientId=682&activeIngredientName=stevia&source=1.
- ↑ List of Permitted Sweeteners. (2012, December 12). Health Canada. Retrieved March 5, 2013, from http://www.hc-sc.gc.ca/fn-an/securit/addit/list/9-sweetener-edulcorant-eng.php
- ↑ Gardana C. , Simonetti P. , Canzi E., Zanchi R., Pietta P. (2003). Metabolism of Stevioside and Rebaudioside A from Stevia rebaudiana Extracts by Human Microflora. Journal of Agricultural and Food Chemistry, 51, 6618-6622.
- ↑ Meddings, J. (1997, May 1).Sucrose--how sweet is it?. Journal of pediatric gastroenterology and nutrition, 24(5), 621-622




