Course:FNH200/Lessons/Lesson 08
Dehydration as a Food Preservation Method
8.0 Overview
Dehydration of foodstuffs involves the removal of water to increase the storage stability of perishable food items. You will learn about processing parameters that affect the ultimate quality of dehydrated plant and animal tissues and fluids that are used as food. You will learn about the principles of spray drying and freeze drying of foods that are amenable to these dehydration methods. The advantages and disadvantages of various dehydration technologies will be discussed. You will learn packaging requirements for maintenance of the quality of dehydrated foods.
Objectives
Upon completion of this lesson, you will be able to:
- illustrate the underlying concepts of various methods of food dehydration
- outline the basis for extension of storage life of foods by dehydration
- compare and contrast methods for dehydrating different foods, and the consequences in terms of food quality
- explain factors affecting the rate of dehydration
- describe the packaging requirements for foods dehydrated by various dehydration methods
8.1 Introduction
Terms to remember |
|
Some reasons for dehydrating foods are:
- preservation of the food (dried milk, juices, fruit);
- retention of the size and shape of the food while imparting storage stability (freeze dried steak, vegetable pieces);
- reducing weight and bulk of food for easier storage and transportation; and
- production of convenience items (instant coffee, instant mashed potatoes, vegetables that rehydrate in instant soup preparations).
Food preservation by dehydration is based on the principle that microbial growth, chemical and enzymatic reactions occur only if sufficient free water is present. When the water activity of foods is lowered there is a direct impact on microbial growth as well as chemical & enzymatic reactions.
Recapping from Lesson 2:
- Water activity (aw) defines the proportion of water in a food that is in the free, unbound form
- Microbial activity, enzymatic activity and chemical reactions can occur only in the free water phase of foods
- Water activity of foods ranges from 0 to 1.0
- Water activity of dehydrated foods is in the range of 0.2 to 0.6
- Microorganisms cannot grow at aw below 0.6
- Chemical reactions (e.g. Maillard browning) can begin to occur at aw of 0.3
| Want to know more? |
| Please visit this website from the Cole Palmer Instrument Company for an overview on water activity, including examples of water activity values for several food products
http://www.foodtechsource.com/rcenter/tech_data/td_water.htm |
It is important to remember that with dehydration, microorganisms are not readily killed. Once the food is rehydrated, microorganisms resume growth if favourable conditions exist.
It is also important to distinguish between food dehydration and concentration, both of which involve the removal of water from foods:
Dehydration implies removal of as much water from the food as possible in order to impart a long storage life.
Concentration, on the other hand, implies that some of the water is removed from the food in order to concentrate the food constituents. Concentrated foods are not inherently shelf-stable and require the use of other forms of food preservation (e.g., refrigeration, freezing, dehydration, thermal processing) to extend storage life.
8.2 Changes in Food during Dehydration
Similar to the other preservation methods we have reviewed, dehydration will cause changes in the food that need to be controlled in order to maintain the highest quality possible. Some of these changes are:
- Cell/tissue Shrinkage. As water is removed from food pieces during dehydration, the cells within the tissue shrink and lose their elasticity. If you have purchased dehydrated vegetables such as carrots, onion slices, or dehydrated fruits such as apple cubes or slices you may have observed the shrinkage that has occurred. Part of the reason for shrinkage of foods that have been dehydrated is that the water migrates from the interior of the food to the surface where it finally evaporates and is carried away by the dehydrating medium. As the water migrates to the surface of the food it carries with it the water soluble substances dissolved in it. The loss of these substances from the interior of the food pieces contributes to the shrinkage observed in dehydrated foods and also contributes to the poor rehydration properties of such foods. Loss of the water soluble components from the interior portions of the food pieces decreases the driving force for attraction of water into the food pieces during rehydration.
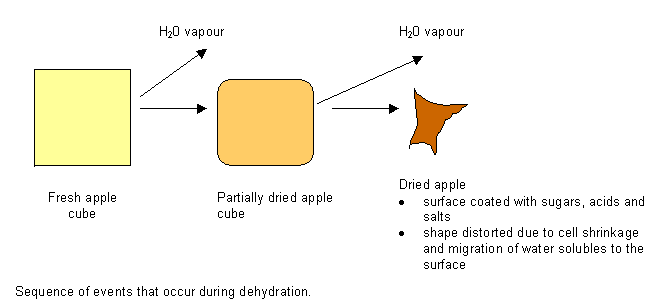
Cell Shrinkage during Dehydration
Sequence of events that occur during dehydration.
(A) Fresh apple cube
(B) Partially dried apple cube
(C) Dried apple surface coated with sugars, acids and salts shape distorted due to cell shrinkage and migration of water solubles to the surface
- Case hardening. Case hardening occurs when rapid drying causes compounds such as sugars to form a hard, fairly impermeable case around the food piece. This phenomenon can cause the rate of dehydration to decrease. Case hardening can occur in high-sugar products such as tropical fruit and many temperate fruit products. Dehydration procedures are designed to minimize the development of case hardening as much possible.
- Chemical changes. A number of chemical changes occur in foods during dehydration in systems employing warm air as the drying agent. The Maillard browning reactions (Lesson 2) cause the development of brown colours and the formation of flavours not originally associated with the fresh product. The Maillard reactions proceed most rapidly when the water content of the food is in the range of 20% down to 15% because the reactants are in very close proximity, which increases the probability of reactions occurring. Thus, drying systems are designed to remove water through the 20-15% range of moisture content as rapidly as possible. This will minimize the negative effects the Maillard reaction has on the flavour of dehydrated food products. The flavour of rehydrated skim milk powder is due largely to the products of the Maillard reaction during dehydration of the milk. Prior to dehydration of egg whites, they are treated with an enzyme, glucose oxidase, which "de-sugars" the egg whites and minimizes the colour and flavour changes that could be caused by the Maillard reactions involving glucose. Poor rehydration can occur because of the loss of the ability of some hydrophilic food constituents to absorb water. Heat denaturation of proteins, starches and gums can decrease the water-holding capacity of dehydrated foods. The salts and sugars concentrated on the outside of the food pieces will dissolve in the water added to the food to rehydrate it. Since those water soluble components are not inside the food pieces, there is less attraction for water to enter the food product. As result, rehydration is less complete. You may have noticed that dehydrated fruit pieces are much sweeter than the fresh fruit. The reason for this phenomenon is that the sugars are concentrated on the outside of the fruit. Loss of volatile substances that contribute to the flavour of foods occurs during dehydration. Generally the higher the drying temperature, the larger the loss of volatiles, with the result that the dehydrated food is less flavourful than the initial product.
Dehydrated foods may show varying extents of shrinkage or chemical changes, depending on the method and conditions used to dry the food.
8.3 Factors Affecting Dehydration
Dehydration of food requires that water (mass) be transferred from the food into the dehydrating environment, and that heat (the driving force that encourages water removal) be transferred to the food to promote water removal from the food. The objectives of food dehydration operations are to dry the food as fast as possible, at the least cost, while creating the fewest changes in food quality.
The composition of the food itself can have an effect of the rate at which dehydration occurs. For example, if water is bound to solutes in the food it will have a lower vapour pressure and therefore will be more difficult to remove. The porosity of the food is also important. Efforts are made to enhance the porosity of foods to be dehydrated in order to facilitate mass transfer and speed of drying rate, thus maximizing the efficiency of dehydration. Porous (sponge-like) structures are formed by creating steam pressure within the product during the drying process. The steam will "puff" the product. Another way of creating porosity is by making a stable foam from a liquid food prior to drying.
In addition to the composition of food, the following factors can also affect heat and mass transfer within food materials undergoing dehydration, and therefore are important to consider in order to control some of the undesirable changes described previously:
- Surface area. It is desirable to maximize the surface-to-volume ratio of the food to be dehydrated to minimize the resistance to heat and mass transfer. Generally, the smaller the food piece, the more rapid the rate of moisture loss.
- Temperature.The hotter the air, the more moisture it will hold before becoming saturated. Drying systems are designed to maximize temperature differences between the product and the drying air to increase the rate of dehydration. An upper limit to drying air temperature is dictated by adverse chemical reactions that can take place in a food at high temperatures. The upper temperature limit is also dictated by the chemical and physical nature of the food.
- Air velocity. The faster the air velocity within a dehydrator, the more rapid the rate of moisture removal. Food dehydrators are designed to maximize the velocity of heated air moving around the food particles to be dried.
- Humidity of the drying air. The drier the air, the more moisture it can absorb before it becomes saturated. The relative humidity of the drying air determines the final moisture content of the food being dried. Knowledge of the equilibrium relative humidity of food is important for the proper design of dehydrators and for the design of packaging systems that will prevent moisture adsorption by the dehydrated food during storage. You may have experienced the loss of crispness of crackers in opened packages during lengthy storage in your cupboards. Crackers have a low equilibrium relative humidity and they tend to adsorb water from the air.
- Atmospheric pressure and vacuum. Water boils at 100°C when it is at a pressure of 1 atm (760 mm Hg). As the pressure lowers, the boiling temperature will decrease. If the temperature is maintained constant, a decrease in pressure will increase the rate of boiling. Some concentrators and dehydrators are operated at pressures below atmospheric pressure in order to increase the rate of boiling and moisture removal. This is especially important in the case of heat-sensitive food products.
8.4 Drying Methods
Sun Drying
Sun drying is mostly used in dry, warm climates. This is a very slow drying method (several days). It is mostly used for fruits, vegetables, and fish. An advantage of this method is the fact that it is quite inexpensive; however, disadvantages include long drying periods (up to several weeks) and the risk of invasion by insects, birds, rodents, and microorganisms.
The appearance is shrunken and has poor rehydration capacity.

Spray Drying
Spray driers are used to produce the greatest quantities of commercially dehydrated foods. Spray driers are restricted to use with liquid foods since the principle of the operation is the introduction of the food as a spray of small droplets into a high velocity stream of warm air. Because droplet sizes are small, drying rates are very rapid and high quality dehydrated food products can be produced. Foods most commonly dehydrated by spray driers include skim milk, coffee, tea and eggs.
You will note that the equipment is designed to maximize drying rate, to produce dry particles of uniform size and to prevent sticking of partially dry food particles to the walls of the spray driers. You should also note that spray driers operate continuously, that is, the product is sprayed into the drying chamber and the dried product and moist air are separated and removed from the driers.

| Want to learn more? |
|
Tray (Air) Drying
Food placed on trays or racks is exposed to heated air at a set velocity. This type of drying can be quite fast and requires heated air with a relative humidity (%RH) lower than that of the product to be dried. Water soluble components migrate to surface of food and are deposited as water evaporates. The dried food has relatively poor re-hydration properties, is shrunken in appearance and is very dense. Products dried this way are: pasta, vegetables, fruit, spices. The diagram below shows the sequence of events that occur during dehydration of fruit tissue.

Drum Drying
During the process of drum drying, food paste is applied to a heated drum in a thin layer to promote rapid drying. As the drum rotates, it picks up a thin film of food material that dries rapidly. The dried food is scraped off the drum near the end of a full rotation of the drum. Dehydrated mashed potatoes and some ready-to-eat breakfast cereals are dried this way. Some popular low fat snack foods and potato chip like products contain drum dried potato flakes as the primary ingredient.

Freeze Drying
Freeze driers are fairly recent innovations as far as food dehydration is concerned. Freeze drying is restricted to high value foods because of the high costs associated with this dehydration method.
During freeze drying, water is removed from food in the frozen state without transition through the liquid state. This phenomenon, called sublimation, is illustrated below:
water(solid) —> water(vapour)
Sublimation of water is accelerated under vacuum conditions. During freeze drying, food is frozen and then placed in the freeze dryer, the dryer is sealed and a vacuum is created and maintained. Application of heat from radiant heaters within the shelves of the freeze drier provides the energy required for sublimation to occur. During freeze drying the amount of heat applied to the food is carefully controlled to maximize the rate of drying without causing transition of water from the solid to the liquid phase. A schematic diagram of a food being freeze dried is shown in Figure 8.5.


During freeze drying, because the food remains rigid during dehydration, the subliming water leaves voids where the ice crystals were located. There is no translocation of water-soluble constituents because there is no movement of liquid, allowing freeze dried foods to retain their shape. Also, freeze dried foods rehydrate almost completely because the voids left by the subliming water provide channels through which water can enter the food, and the water-soluble components of the food in their original locations provide the driving force for rehydration. Freeze dried foods do not usually exhibit the shrinkage and chemical changes noted earlier to occur in other dehydrated foods.
- If you have consumed the instant soup mixes that are prepared in a cup with the addition of boiling water, you will have noted that the vegetables have rehydrated within several minutes and that they possess a fresh flavour. Those vegetable pieces were freeze dried.
- You may wish to compare the rate and extent of rehydration of vegetable pieces in an instant soup mix (in which vegetables were freeze dried) with those vegetables from a soup mix which has to be boiled for about 10 minutes in order to rehydrate the vegetable pieces (these vegetable pieces were tray-air dried).
Vacuum Microwave Drying
Vacuum microwave or radiant energy vacuum (REV) technology is being developed for the dehydration of food, nutraceutical and pharmaceutical products by Dr. Tim Durance in the Food Science program at the University of British Columbia. The technology consists of a combination of vacuum (in order to keep the temperature low) and microwaves (for ultra-rapid energy transfer), producing high quality products with less nutrient loss, better flavour retention, and less colour change. Vacuum microwave dried (VMD) products retain a more natural appearance and have the advantage of complete re-hydration (reconstitution). VMD is a quick drying method when compared to freeze drying and air drying methods (VMD can take only a few minutes, compared to hours in freeze drying).
For more information, visit http://www.enwave.net/ (Links to an external site.)
Deep Fat Frying
During deep fat frying, the high temperature of hot oil causes water in the food to evaporate rapidly, often accompanied by substantial pick up of oil by the food being dried. Dried foods such as potato chips have a low water content (as well as a low water activity) but a high oil content. Many snack foods and bakery products, such as donuts, are produced by means of deep fat frying.
Do you know how instant noodles are made? After cooking and shaping, the noodles are dried either by deep-fat frying or non-frying (hot-air) drying. Frying is usually done at 140-150°C for 1-2 minutes, while hot-air drying uses a temperature of about 80°C for 30 min. The two drying methods yield products that differ greatly in fat content.
Extrusion (cooking) Drying
Slurry of food is passed though a tube, under pressure, that is heated by steam. The moist heat causes starch gelatinization and cooking of the product. Product is forced though a narrow opening (a die which can produce a product with a variety of shapes) at the end of the tube and escaping steam causes the dehydrating product to puff. Many ready-to-eat breakfast cereals and snack foods are produced this way.

Common food products prepared by extrusion drying
| Category | Examples |
|---|---|
| Ready-to-eat breakfast cereals | Puffed cereals, flaked cereals, high-fiber strands |
| Snacks | Puffed snacks, Crispbreads |
| Confections | Licorice, some chocolates |
| Texturised protein | Soy meat-analogues, "processed" cheese |
| Infant foods | Biscuits, weaning cereals |
| Want to learn more? |
|
8.5 Packaging Requirements for Dehydrated Foods
Many dried foods also require that the packaging material provide physical protection to prevent the food from becoming crushed during distribution and handling.
| Want to learn more? |
Take a look at dehydrated products in your kitchen cupboard or in the grocery store, and think about:
|
8.6 Summary of Lesson 8
- Preservation of food by dehydration involves the removal of water (thus lowering the water activity) from the food to extend the food's shelf life by slowing down microbial growth and chemical/enzymatic reactions.
- Microbial growth and chemical/enzymatic reactions will resume once the food is re-constituted or re-hydrated.
- During dehydration of food, changes such as "cell shrinkage, case hardening, and different chemical changes", can take place.
- During dehydration, several factors (e.g. temperature, air velocity, humidity of the drying air, etc) must be controlled in order to prevent undesirable changes (case hardening, excessive cell shrinkage, etc)
- Packaging materials should not only impart physical protection, but also assist in preserving dehydrated foods by further protecting against moisture absorption, as well as preventing interactions with oxygen and light.
Supplemental Video: Extrusion Drying
Authorship:
FNH 200 Course content on this wiki page and associated lesson pages was originally authored by Drs. Brent Skura, Andrea Liceaga, and Eunice Li-Chan. Ongoing edits and updates are contributed by past and current instructors including Drs. Andrea Liceaga, Azita Madadi-Noei, Nooshin Alizadeh-Pasdar, and Judy Chan.
|
|
