Symmetry and Group Theory
Symmetry and Group Theory are an effective way of simplifying complex problems and determining how best to evaluate a particular spectroscopic problem. The uses of group theory are very general but our focus will be on the application of group theoretical principals to help us evaluate and utilize spectroscopy in inorganic chemistry.
Lecture Notes for Group Theory
Symmetry Elements and Symmetry Operations
Improper Axis of Rotation ( )
The improper axis of rotation is a combination of two simpler operations: a rotation (about the appropriate axis) followed by a reflection through the plane ( ) that is perpendicular to the rotation axis (need graphic here). In the event where , then operations are performed sequentially as follows:
Remember that operations are performed sequentially from right to left!
In an Abelian group (where operations are commutative as well as associative), we can see that the two operations will cancel themselves out, allowing us to easily determine that . We also find that although in all cases, the same is not true for improper axes of rotation, i.e., .
Symmetry Point Groups
Molecules can be classified based on their symmetry point groups. Although there are a large number of different point groups, they are generally classified in four general classes depending on the number and type of rotational elements that can be used to describe a particular molecular geometry:
| Point Group | Category | Molecular Structure | User | Details |
|---|---|---|---|---|
| C1 | Low Symmetry | 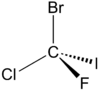 |
PK | Add specific text that describes any restrictions placed on the molecule (none in this case). Also include reference here if appropriate - use DOI links wherever possible (e.g. doi:10.1021/ja992003l). |
| C2v | Rotational | ![Caption: [dpp-nacnacCF3]Rh(phdi) where [dpp-nacnacCF3]− = CH[C(CF3)(N-iPr2C6H3)]2− and phdi = 9,10-phenenthrenediimine](http://wiki.ubc.ca/images/thumb/c/c6/Rh.png/100px-Rh.png) |
Scott Ryken | I am making the assumption that the nacnac ligand is completely planar, including the diisopropylphenyl groups and I am ignoring hydrogen atoms. This is also a square planar complex. ( DOI: 10.1021/ic1007632). |
| D4h | Dihedral | 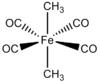 |
Scott Ryken | Protons are being ignored and it is a octahedral in geometry, not in symmetry. I proposed this example and I don't know if it has been made. I assume it probably has been. |
| Ci | Low Symmetry | 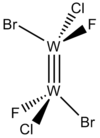 |
Scott Ryken | This is a theoretical compound based on other Tungsten dimers but manipulated to have only an inversion center. ( DOI: 10.1021/ja00431a025) |
| D5h | Dihedral | 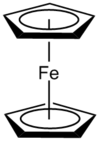 |
Jtherrien | Ferrocene in an eclipsed conformation. |
| D5d | Dihedral | 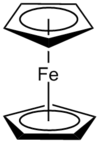 |
Jtherrien | Ferrocene in a staggered conformation. |
| C4v | Rotational | 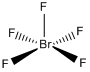 |
Jtherrien | DeKook, R. L.; Gray, H. B. Chemical Structure and Bonding, 2nd ed.; University Science Books: Sausalito, California, 1989; p. 110. |
| Td | High Symmetry | 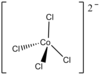 |
Scott Ryken | There is a 2 minus charge and any counter ions that are being ignored so there is nothing affecting the tetrahedral geometry. |
| Cs | Low Symmetry | 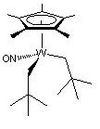 |
RBaillie | Protons have been ignored. The Cp* ring is positioned with a methyl group evenly between the neopentyl ligands. The structure shown is the structure in solution rather than the solid state molecular structure. [2] |
| C1 | Low Symmetry | 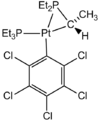 |
Joe Clarkson | dx.doi.org/10.1021/om1010879 |
| C2 | Rotational |  |
User:Rhett Baillie | more stuff |
| C3v | Rotational | Joe Clarkson | more stuff | |
| D3h | Dihedral | 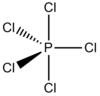 |
Joe Clarkson | more stuff |
| D3 | Dihedral | 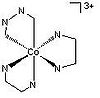 |
RBaillie | Protons are ignored. Any counterions are ignored. |
| Th | High Symmetry | 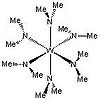 |
User:Rhett Baillie | [3] |
| Oh | High Symmetry | 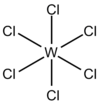 |
Joe Clarkson | more stuff |
| Ih | High Symmetry | 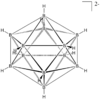 |
Jtherrien | The counterion (commonly caesium) is ignored. doi: 10.1021/jp200627f |
| D2h | Dihedral | 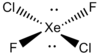 |
JanetOchola | more stuff |
| C3 | Rotational | 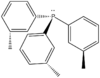 |
JanetOchola | Ignored the protons |
| C2h | Rotational | 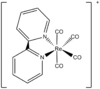 |
JanetOchola | Ignored the protons and the bpy group is flat on the x-y plane. Ignored the positive charge. doi: 10.1021/ic00126a031 |
| D4d | Dihedral | JanetOchola | Crown conformation | |
| C5v | Rotational |  |
DanHu | none |
| D2d | Dihedral | 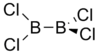 |
DanHu | none |
| Td | High Symmetry | 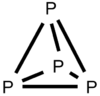 |
DanHu | Tetrahedron |
| Cs | Low Symmetry | 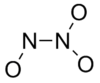 |
DanHu | none |
| C∞v | Rotational | 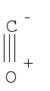 |
AlyssaYeo | |
| D∞h | Dihedral | AlyssaYeo | ||
| C3h | Rotational | 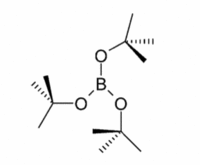 |
AlyssaYeo | doi:10.1002/zaac.200600229 |
| S4 | Rotational | ![Caption:[Fe(SMe)4]-](http://wiki.ubc.ca/images/thumb/a/ae/Fe%28SMe%294.gif/200px-Fe%28SMe%294.gif) |
AlyssaYeo | ORTEP diagram of the anion of [Et4N][Fe(SMe)4] (view down the crystallographic S4 axis) doi: 10.1021/ic00048a029 |
| S6 | Rotational | 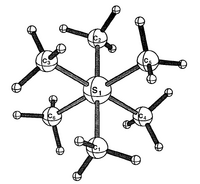 |
Clement Cheung | Assumes the methyl groups are rotated in the same direction on all 6 C-S bonds ( doi:abs/10.1021/ic940240d ) |
| D6h | Dihedral | 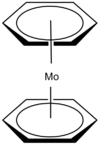 |
Clement Cheung | The two benzene each donates 6 electrons and the carbons directly overlap each other in the two rings |
| Td | High Symmetry | 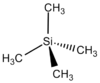 |
Clement Cheung | Assumes the absence of protons |
| C1 | Low Symmetry | 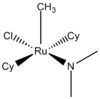 |
Clement Cheung | Assumes that the transition state of the transition metal catalyst does not revert to the trigonal bipyramidal shape with rearrangement of the ligands and that the N-methyls are not in the equatorial plane |
| D6d | Rotational | 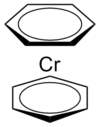 |
Hessam Mehr | Staggered conformation assumed (more information available here). |
| D8h | Rotational | 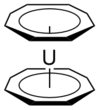 |
Hessam Mehr | Eclipsed conformation assumed (from Wikipedia). |
| C6v | Rotational | 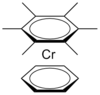 |
Hessam Mehr | Hydrogen atoms ignored). |
Properties of a Mathematical Group
Identity: There is an element of the group such that • = • = , for any element of the group.
Closure: If and are in the group then the result of • is also a member of the group.
Inverse: For any element of the group, there is an such that • = • = .
Associativity: If , and are in the group then • • = • • .
Representations of Groups: Character Tables
Simple Groups: , , .
Groups: , , , , , , .
Groups: , , , , , , .
Groups: , , , , , , .
Groups: , , , , , , .
Groups: , , , , , , .
Groups: , , , , , , .
Groups: , , , , , .
Cubic Groups: , , , , , , .
Linear Groups: ,
Examples in Chemistry where Symmetry and Group Theory are commonly utilized
- Crystallography
- Isomers
- NMR equivalency
- Determining spectroscopic/photochemical selection rules (electronic, angular momentum etc.)
- IR/Raman activity
- Determining the nature of atomic and molecular orbitals
- Symmetry labels in molecular orbital diagrams
- Determining structures of compounds (e.g. tetrahedral, octahedral etc.)
- Predicting reactivity:
- e.g. forbidden and allowed transitions states for pericyclic reactions
- predicting, or rationalizing stereochemical outcome of a reaction
Literature examples of the use of symmetry/group theory in inorganic chemistry
"Resonance Raman spectroscopy as a probe of the bis(mu-oxo)dicopper core", Holland PL, Cramer CJ, Wilkinson EC, Mahapatra S, Rodgers KR, Itoh S, Taki M, Fukuzumi S, Que L, Tolman WB, J. Am. Chem. Soc., 2000, 122(5), 792-802, doi:10.1021/ja992003l. PK
- This article explores the possible vibrational modes of dicopper bis(mu-oxo) complexes as a function of the symmetry of the complexes. Significant differences can be observed as a function of the symmetry of the dimetallic core, which can be easily explained by group theoretical analysis. PK
"A multiplet analysis of Fe K-edge 1s->3d pre-edge features of iron complexes", Tami E. Westre, Pierre Kennepohl, Jane G. DeWitt, Britt Hedman, Keith O. Hodgson, and Edward I. Solomon, J. Am. Chem. Soc., 1997, 119(27), 6297-6314, doi:10.1021/ja964352a.
- This article develops a group theoretical and ligand field analysis of the pre-edge features for Fe K-edge X-ray sbsorption spectroscopy. The overall analysis is completely based on group theory to understand both the bonding and spectroscopic selection rules that apply for this spectroscopic method. PK
"Transition Metal Containing Decatungstosilicate dimer [M(H2O)2-(SiW10O35)2]10- (M = Mn2+, Co2+, Ni2+)", Bassil BS, Dickman MH, Reicke M, Kortz U, Keita B and Nadjo L, "Dalton Trans.", "2006", 35, 4253-4259, doi:10.1039/b606911h.
- These authors synthesized new tungstosilicate dimers with C2v point group symmetry. They talk about how the compounds with different metal ions were all the same symmetric C2v dimers and they all crystallized in the same space group as well. Kimosten
"Low-Valent Ruthenium Complexes of the Non-innocent 2,6-Bis(imino)pyridine Ligand", Gallager, M, Wieder NL, Dioumaev, VK, Carrol, PJ, Berry, DH. "Organometallics" doi:10.1021/om9009075
- This article explores the synthesis and characterization of a Ru(0) 2,6-Bis(imino)pyridine dinitrogen compound. Group theory is used to geometrically describe the N2 compound. A Nujol IR spectrum is taken to attempt to characterize the degree of N2 activation. The IR stretch assigned to the N2 bond is observed as a weak signal at 1851 cm-1. The idealized geometry that the authors use to describe the compound (D2d) should not allow the N2 bond to be IR active. TrumanWambach
"Construction of nano- and microporous frameworks from octahedral bubble clusters", S. M. Woodley, M. B. Watkins, A. A. Sokol, S. A. Shevlin and C. R. A. Catlow, Phys. Chem. Chem. Phys, 2009, 11, 3176-3185, doi:10.1039/b902600b
- This article describes a method of constructing microporous frameworks using eight different high symmetry ZnO clusters as building blocks. The building blocks have either T, Td, Th or O point group symmetry. The lattice energies of the final structures are calculated using interatomic potentials and it is determined that the frameworks consisting of clusters with Th point group symmetry are much more stable than those with T, Td or O symmetry. (AshleeHowarth)
"Synthesis of Pincer-Type Bis(benzimidazolin-2-ylidene) Palladium Complexes and Their Application in C-C Coupling Reactions", F. Ekkehardt Hahn, Mareike C. Jahnke, Tania Pape, Organometallics, 2007, 26, 150-154. doi:10.1021/om060882w
- This article focuses on the preparation and catalytic properties of palladium pincer N-heterocyclic carbene complexes. In the 1H NMR spectroscopy analysis of the Pd compound, temperature dependent studies are undertaken to analyze the thermodynamic parameters of the atropisomerization process which the ligand backbone undergoes. At higher temperatures, an averaged structure with C2v symmetry is observed. Lwence
“Why ‘spherical’ cyclophosphazenic dandelion dendrimers have a dipole moment?” Fayet J-P, Sournies F, Crasnier F, Labarre M-C, Labarre J-F, Main Group Chem. 1997, 2(2), 107-110, doi:10.1080/10241229712331341224
- This article provides an explanation to why “spherical” cyclophosphazenic dendrimers consist of dipole moments when their geometrical morphology appears to be highly symmetrical. Peraminolysis of N3P3Cl6 (which possesses D3h symmetry) by 1,6-diaminohexane generates dendrimers of C3 symmetry, thus suggesting presence of a dipole moment. By molecular modeling and measurements in dipole moments, the decrease in symmetry of the dendrimer is found to be due to the non-symmetrical distribution of electron density of the nitrogen atoms of the amino groups, which is responsible for the significant dipole moments measured in the dendrimers. ReneeMan
"Application of Symmetry Operation Measures in Structural Inorganic Chemistry", Jorge Echeverria and Santiago Aivarez, Inorg. Chem., 2008, 47(23), 10965-10970, doi:10.1021/ic801264n.
- This article details the use of applying symmetry operation measures to describe distortions in octahedral and tetrahedral crystallographic sites, as well as, to show the Bailar and tetragonal Jahn-Teller distortions of molecular transition metal complexes. The symmetry operation measure (Z(R)) is a numerical indicator which shows if a certain structure has a given symmetry operation (R). The novelty prescribed in this paper is the use of only four symmetry operation (C2, C3, C4 and i) to fully differentiate from different symmetry subgroups which corresponds to certain types of distortions. JackyYim
"C60-derived nanobaskets: stability, vibrational signatures, and molecular trapping", SG dos Santos, M S Pires, V Lemos, V N Freire, E W S Caetano, D S Galvao, F Sato and E L Albuquerque, Nanotechnology, 2009,20 (39): 395701. doi 10.1088/0957-4484/20/39/395701
This article uses simulations to investigate C60-derived nanobaskets obtained by effecting planar cuts in the atomic cage of fullerene. Infrared selection rules for these baskets are used to predict their symmetries and thus provide a method of differentiating between the nanostructures. Three baskets with different molecular formulae are investigated: C40H10(C5v), C39H12 (3v) and C46H12 (C2v). SusanVickers
“A novel hexachelating amino-thiol ligand and its complex with gallium(III)” Dennis A. Moore, Phillip E. Fanwick, Michael J. Welch, Inorg. Chem. 1990, 29(4), 672-676, DOI: 10.1021/ic00329a022
- This work describes the chelators I ,4,7-Tris( 2-mercaptoethy1)-1,4,7-triazacyclononane (TS-TACNH), and 1,4,7-Triazacyclononane-l,4,7-triaceticacid (NOTA) bound to gallium. Crystal structures are analyzed and found to be present in the centric P21/n unit cell. Both the delta and lambda enantiomers are formed for reach ligand-metal complex. This is a simple paper describing the synthesis and characterization of these complexes and their potential use in radiochemistry and nuclear medicine. The binding of these ligands with gallium(III) was expected to be very similar to iron(III) (trigonal prismatic geometry); however the higher affinity of gallium(III) for nitrogen coordination resulted in closer to octahedral geometry.
"Structure and Vibrational Spectra of Ti(IV) Hydroxides and Their Clusters with Expanded Titanium Coordination. DFT Study", Ignatyev IS, Montejo M, Gonzalez JJL, J. Phys. Chem. A., 2007, 111(32): 7973-7979. DOI: 10.1021/jp073423x
- The most stable equilibrium structures of H4-nTi(OH)n (n=2-4) molecules and Ti(OH)4 clusters were determined using computational chemistry at the 6-31+G(d) basis set. Theoretical vibrational (IR) frequencies of TiO stretching modes were compared to experimental IR vibrational frequencies. The point groups of Ti(OH)4 dimers, trimers and Ti3O12 molecules are discussed along with their vibrational frequency & symmetry relationships. AlexandraAnderson
"Consideration on the symmetry of loop order in cuprates", A. Shekhter, C.M. Varma. Phys. Rev. B, 2009, 80, 214501, doi:10.1103/PhysRevB.80.214501.
- This article examines the effects of magnetic fields on the symmetry of cuprate. The changes in symmetry are then considered for the cuprates' psuedo gap phase and other parameters that make cuprates interesting. AmberJuilfs
"Symmetry: A guide to its application in 2D electron crystallography", Landsberg MJ, Hankamer B. J. Struct. Biol., 2007, 160(3): 332-343, doi:10.1016/j.jsb.2007.07.002.
- This mini-review sets out to summarize all aspects that define 2D crystallographic symmetry as applied to the study of macromolecular structure. It provides a solid basis allowing for the accurate identification of symmetry and the subsequent application of symmetry based averaging in structure refinement. CuilingXu
"Hangman Corroles: Efficient Synthesis and Oxygen ReactionChemistry", Dilek K. Dogutan, Sebastian A. Stoian, Robert McGuire, Jr., Matthias Schwalbe, Thomas S. Teets, Daniel G. Nocera J. Am. Chem. Soc., 2011, 133(1), 131-140, doi:10.1021/ja108904s.
- This paper discusses a new synthesis of Hangman Corroles,a type of corrin macrocycle, and the reactivity of the resultant cobalt complexes towards oxygen. Symmetry and group theory are prevalent throughout this paper from the discussion of crystallography to EPR transitions. In particular, DFT calculations were preformed with symmetry constraints and then further optimized without symmetry constraints. The symmetry constraints allows for faster initial optimization, and the fact that the calculations generate the same results when symmetry constraints were removed suggests that these symmetry elements are present in the molecule. FraserPick
"Vibrational frequencies and structural determination of phosphorus tricyanide", James O. Jensen, Spectrochemica Acta Part A, 2004, 60, 2537-2540, doi:10.1016/j.saa.2003.12.032.
- This paper describes the use of theoretical calculations of the vibrational frequencies of phosphorus tricyanide, and how these calculated values were used to assign the experimental values to the corresponding stretch. The calculations were performed using the C3v symmetry of phosphorus tricyanide. AndrewPriegert
"Phthalocyanine as a Chemically Inert, Redox-Active Ligand: Structural and Electronic Properties of a Nb(IV)-Oxo Complex Incorporating a Highly Reduced Phthalocyanine(4-) Anion", Edwin W.Y. Wong, Charles J. Walsby, Tim Storr, Daniel Leznoff Inorg. Chem., 2010, 49, 3343-3350, [4].
- This paper investigates the electronic structure of a reduced niobium(V) phthalocyanine complex. The electronic structures of the parent complex and reduced forms were compared via UV-Vis absorption, X-ray crystallography, EPR, ENDOR, and TD-DFT. The symmetry of each complex was used to predict changes in the UV-Vis spectra from parent to reduced forms, and the line shape of the EPR spectra was predicted by the observed symmetry of the complexes. CaterinaRamogida
"New C2v- and Chiral C2-Symmetric Olefin Polymerization Catalysts Based on Nickel(II) and Palladium(II) Diimine Complexes Bearing 2,6-Diphenyl Aniline Moieties: Synthesis, Structural Characterization, and First Insight into Polymerization Properties", Markus Schmid, Robert Eberhardt, Martti Klinga, Markku Leskela and Bernhard Rieger Organometallics, 2001, 20,2321-2330, [5].
- The paper discusses the synthesis and characterization of Novel olefin polymerization catalysts with C2v and C2 symmetry. The discussion indicates how sterics affect the geometry and the symmetry of metal complexes. DineshAluthge
"Activation of Methane by Zinc: Gas-Phase Synthesis, Structure, and Bonding of HZnCH3", Michael A. Flory, Aldo J. Apponi, Lindsay N. Zack, and Lucy M. Ziurys. J. Am. Chem. Soc., 2010, 132, 17186–17192, doi:10.1021/ja106121v.
- The authors synthesized MeZnH in the gas phase and identified it using rotational spectroscopy. Its spectral features, particularly the existence of a K-ladder, indicate that the molecule has symmetry; analysis of the rotational constants of the isotopologues allowed determination of its bond lengths and angles. CatherineChow
"LiCoO2 Concaved Cuboctahedrons from Symmetry-Controlled Topological Reactions" Chen, H.; Wu, L.; Zhang, L.; Zhu, Y. Grey, C. J. Am. Chem. Soc. 2010, 133, 262-270. [6]
- The growth of novel cuboctahedron nanoparticles is presented. The unique structure adopted by these nanoparticles is controlled using symmetry as a synthetic tool. X-ray diffraction and other analytical methods are employed in order to determine how and why these marvelous crystals form. Peter Christensen
-
Chen et al. [1]
"Molecular Dials: Hindered Rotations in Mono- andDiferrocenyl Anthracenes and Triptycenes" Nikitin,K; Muller-Bunz, H; Ortin, Y; Muldoon, J; McGlinchey, M. J. J. Am. Chem. Soc. 2010, 132, 17617-17622. [7]
- Interesting mono- and diferrocenyl anthracenes and triptycenes have been synthesized. NMR methods (including 2D-EXSY NMR) are employed to probe into the hindered rotational behaviors of these complexes under different temperatures, on the basis that molecules with certain symmetry (e.g. and ) yield specific signals. Yang Cao
"A New Set of Structurally Related Enantiopure Polypyrazolyl Ligands of Varying Rotational Symmetry: Synthesis, Metal Complexation, and Comparison of Asymmetric Induction" Michael C. Keyes, Bradley M. Chamberlain, Scott A.
Caltagirone, Jason A. Halfen, and William B. Tolman. Oragnometallics. 1998, 17, 1984-1992. [8]
- This paper discusses the synthesis of various enantiomerically pure ligands with C1, C2, or C3 symmetry. These ligands were used to form copper complexes to perform cyclopropanation of styrene. Their study showed that the complexes with C3 symmetry had significantly higher enantioselectivity compared to the ligands with C1 or C2 symmetry. PhillipTaylor
"Arylene Imine Macrocycles of C-3h and C-3 Symmetry from Reductive Imination of Nitroformylarenes" Andrew L. Korich and Thomas S. Hughes. ORGANIC LETTERS 2008, 23, 5405-5408. [9]
- The author synthesized some kinds of Schiff base macrocycles starting from nitroaldehyde precursors. In this method, each imine macrocycle can be traced back to a single fragment that contains both the amine and the carbonyl moieties. All of those kinds of macrocycles are in C3 symmetry, which is evident by the simplicity of the 1H NMR spectra. Zhengyu Chen
"Identification of a Novel η 2 -Se2 Bonding Mode in Cu(I) Complexes of the Dimeric Selenocarbonyl Dianions, [(EPh2P)2CSeSeC(PPh2E)2 [ 2- (E = S, Se)" Maarit Risto, Jari Konu, and Tristram Chivers INORGANIC CHEMISTRY 2011, 50, 406-408. [10]
- The author synthesized and characterized some Cu(I) complexes with novel η 2 -Se2 bonding mode. X-ray crystallography shows different symmetry operations among three different complexes: 1-x,1-y,1-z, 2-x,1-y,1-z, and 1-x,1-y,1-z, respectively. Jiazhang Wang
"Nanocrystal Materials with Modified Symmetry" A. M. Zheltikov Laser Physics 2001, 11(9), 1024-1028. []
- The author provides a qualitative analysis of birefringence and changes in linear and nonlinear-optical properties of porous materials based on crystal symmetry considerations. Joanna De Witt





















































































![Chen et al. [1]](http://wiki.ubc.ca/images/thumb/5/5e/Cuboctahedron.jpg/82px-Cuboctahedron.jpg)