LFS:SoilWeb/Soil Components/Soil Air
Soil air is the gaseous phase of the soil. Soil air plays an important role in plant growth and the activity of soil organisms. Soil pores are filled with air and water, and there is a dynamic equilibrium between water and air content within a soil. When water enters the soil, it displaces air from some of the pores; hence, the air content of a soil is inversely related to its water content.
Proportions of soil components (on a volume basis) under different plant growing conditions:
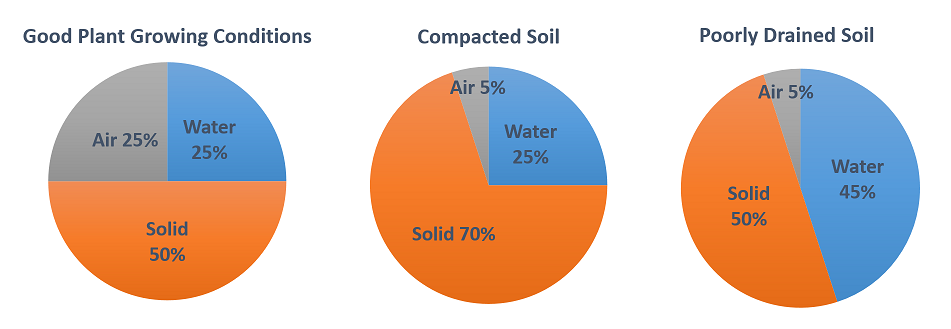
The amount and composition of air in soil are dynamic and to a large degree are determined by water content and activity of soil organisms. The main constituents of soil air, as in the atmosphere, are nitrogen, oxygen, and carbon dioxide. However, the composition of soil air is different from that of the atmosphere because it cannot readily mix with air above the soil. Soil air has higher moisture content than the atmosphere, with relative humidity approaching 100% under optimum conditions (humidity is not as variable in soil as it is in the atmosphere).
Composition of soil air and atmosphere:
- Nitrogen: Soil Air (79.2%) Atmosphere (79.0%)
- Oxygen: Soil Air (20.6%) Atmosphere (20.9%)
- Carbon Dioxide: Soil Air (0.25%) Atmosphere (0.03%)
The metabolic activity of plant roots, microbes and soil fauna all affect the composition of soil air, since oxygen is required for respiration of both plant roots and soil organisms, while carbon dioxide is a product of respiration of plant roots and soil organisms. In some cases soil air may also contain the pollutant gases (e.g., radon) that might be picked up from the soil underneath a building and carried by air leakage into the building.
Another interesting point about soil air and its composition is related to the climate change. Soils play an important role in climate change because they can serve as both a source and sink for greenhouse gases, especially carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O). These gases are generated as natural by-products of soil microbial processes, including some of the same processes that provide plant-available nutrients and contribute to ecosystem stability (such as respiration and nitrification). However, if the equilibrium of these microbial processes is disrupted by a changing climate, the rate of greenhouse gas emissions could change rapidly.
CO2 chambers are used to measure soil respiration (Photos: Biometeorology Group, UBC):
Movement of Gases within Soil
Gas molecules in the soil are in continuous thermal motion according to the kinetic theory of gases. The exchange of gases between the atmosphere and soil, and within the soil, is facilitated by two mechanisms:
- Mass flow (convection) of air - the moving force is a gradient of total gas pressure, and it results in the entire mass of air streaming from a zone of higher pressure to one of lower pressure. Mass flow of air is much less important than diffusion, except perhaps in layers at or very near the soil surface.
- Diffusion - moving force is gradient of partial pressure of any constituent member of air to migrate from a zone of higher to lower pressure, even while air as a whole may remain stationary. In other words, through diffusion each gas moves in a direction determined by its own partial pressure.
The oxygen flux density due to diffusion is proportional to the oxygen concentration gradient along the axis, and the proportionality factor is called the (oxygen) diffusion coefficient (D). This statement is an example of Fick's Law of Diffusion, which can be expressed as follows:
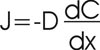
where J is the diffusive flux density of the gas (oxygen in this example) (mg/m2/s) along the x-axis, C is oxygen concentration in the soil air (units are g/m3), x is distance along x-axis (m), dC/dx is the oxygen concentration gradient (g/m3), and D is the (oxygen) diffusion coefficient (m2/s).
The oxygen diffusion coefficient (D) for diffusion in air is about 10,000 times as large as the coefficient for diffusion in water. Thus the oxygen diffusion coefficient (D) of a soil is very strongly influenced by three factors:
- Air-filled porosity (Va/Vt), which decreases with increasing soil water content.
- The continuity of air-filled pores, which decreases with increasing soil water content.
- The tortuosity of air-filled pores, which increases with increasing soil water content.
Growth of most plants and survival of their roots normally requires maintenance of adequate soil oxygen. This in turn requires maintainance of soil water well below saturation, to enable rapid gas diffusion in the soil.
Soil Porosity
Soil porosity (f) is the ratio of pore volume (Vf) to total soil volume (Vt):
| f = Vf / Vt |
Soil porosity of mineral soil horizons at or close to the surface is generally between 30 and 60%. Porosity of subsurface soil is lower than in surface soil due to compaction by gravity.
Porosity tells us nothing about the relative amounts of large and small pores, and should be interpreted with caution. Generally, high porosity (e.g., 60%) is an indicator of lack of compaction and good soil conditions.

