LFS:SoilWeb/Soil Components/Organic Components
Soil organic matter (SOM) can be of plant, animal, or microbial origin and may be relatively fresh or highly decomposed and transformed. In the APBI 200 - Introduction to Soil Science course, the terms "soil organic matter" and "humus" are considered synonyms.
One of many definitions of soil organic matter states that, "it is a complex and rather resistant mixture of brown or dark brown amorphous and colloidal organic compounds that result from microbial decomposition and synthesis and has chemical and physical properties of great significance to soils and plants".
Many species of fungi and bacteria decompose soil organic matter. The fungi and bacteria that can break down the woody tissues and cellulose of intact leaves, stems and dead roots of plants perform the function of decomposition of organic matter that often builds humus and returns nutrients back to the soil. When the species that are capable of decomposition are lacking, leaves and other organic matter remain intact and the food energy they contain are not released to drive the food web.

Decomposition of wood (Photo: Dr. S. Grayston, UBC)
RELATED LINK:
|
Soils may vary greatly in their organic matter contents. For example, a typical prairie grassland soil (Chernozem) may contain 5-6% organic matter (by weight) in surface horizons, sandy desert soil may have <1% of organic matter, while Organic soils (by definition) contain >30% SOM (by weight).

A profile of a Black Chernozem showing accumulation of organic matter in the surface horizons, indicated by the dark brown colour (Photo: Dr. Maja Krzic, UBC).

A profile of an Organic soil showing more than 2 metres of accumulation of organic matter (Photo: Dr. Maja Krzic, UBC).

A simple test that allows qualitative comparison of soil organic matter content.
Organic matter is an important soil component because it:
- holds soil particles together and stabilizes the soil, thus reducing the risk of erosion;
- aids crop growth by improving the soil's ability to store and transmit air and water;
- stores and supplies many nutrients needed for the growth of plants and soil organisms;
- prevents or minimizes soil compaction;
- retains carbon from the atmosphere;
- reduces the negative environmental effects of pesticides, heavy metals, and many other pollutants.
Non-humic Substances
The physically and chemically heterogeneous mixture of materials that make up SOM varies substantially in terms of quantity and resistance to biological decomposition.
SOM includes primary components that are inherited from plant and animal residues entering the soil. Primary components are sometimes referred to as non-humic substances. These compounds are relatively easily decomposed by microorganisms and they persist in soil for a brief time (e.g., several months or years). They make about 20-30% of total SOM.
These include:
- Carbohydrates and several derivatives (monosaccharides and polisaccharides such as cellulose, hemicellulose)
- Amino acids and several derivatives (proteins)
- Lignin (condensed polyphenil-propane structures of extremely large molecular weight, very resistant to microbial decomposition)
- Lipids (a class of organic compounds that is a convenient analytical group rather than a specific type of compound. They include fats, oils, waxes, phospholipids, and resins)
- A variety of other compounds
RELATED LINK:
|
Humic Substances

SOM also includes secondary components, compounds formed within the soil by breaking down organic structures and synthesizing new ones. The secondary components include carbohydrates, amino acids, lipids and others, more or less resembling many primary components. In addition, secondary components also include humic substances, which are rather different from most primary components.
Humic substances are products of biochemical decomposition. They are complex substances of high molecular weight, which are resistant to further decomposition. Consequently they tend to accumulate in the soil. Most are dark and are hence responsible for the dark soil color that is commonly associated with soils of high organic matter content. Humic substances make up 60-80% of total SOM.
Humic substances can be classified based on their behavior in acids and bases. There are three general groups of humic substances: (1) fulvic acids, (2) humic acids, and (3) humin. These three groups are nondescript mixtures of many chemical compounds and should not be considered as distinct chemical entities.
- Humins are particles with large molecular weight, relatively small specific surface area, relatively low number of carboxyl groups, and are inactive.
- Humic acids are smaller in size than humins (approximately colloid-sized) and have more carboxyl groups than humins.
- Fulvic acids are the smallest in size among humic substances, and have a large number of carboxyl groups per unit mass. For this reason they are the most active among humic substances.
The humic substances possess carboxyl groups (*R-COOH), which give them their acidic character and make them effective in buffering soil pH:
R-COOH ↔ R-COO- + H+
|
In a common range of soil pH the overall charge on SOM is negative, due to dissociation of hydrogen ions from carboxyl and other functional groups.
The percentage of the humus which occurs in the various humic fractions varies considerably from one soil type to another. The humus of forest soils is characterized by a high content of fulvic acids, while the humus of peat and grassland soils is high in humic acids (see figure). The humic acid:fulvic acid ratio usually, but not always, decreases with increasing depth.
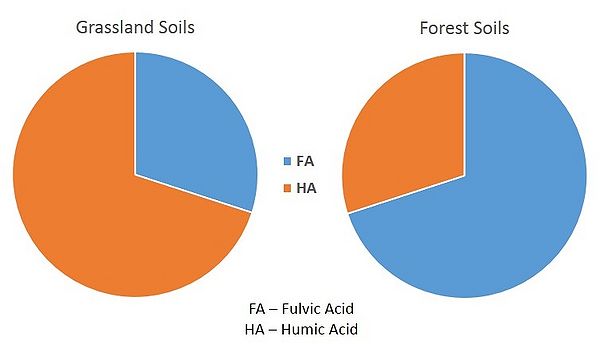
Podzols and Luvisols (forest soils), predominantly have HA:FA <1. HA found in these soils have a small extent of aromatic rings condensation, which makes them similar to FA. Considerable hydrophilic properties of HA allow them to chelate various polyvalent cations. These HA chelates are quite mobile and tend to move to deeper parts of soil profile. Considerable mobility of these HA chelates favours the soil formation process called podzolization (translocation of Fe/Al oxides and hydroxides within soil profile).
In Chernozems (grassland soils) and some Luvisols, HA:FA >1. The extent of aromatic ring condensation is high in these HAs, which causes their hydrophobic properties and inability to create chelates. These HA are strongly connected with mineral soil particles, which reduces mobility of these HA.
RELATED LINK:
|