LFS:SoilWeb/Interactions Among Soil Components/Soil Acidity
The soil pH is a measure of the acidity or basicity in soils.
Generally, pH is defined as the negative logarithm (base 10) of the activity of hydrogen ions (H+) in a solution. pH ranges from 0 to 14, with 7 being neutral. A pH below 7 is acidic and above 7 is basic.
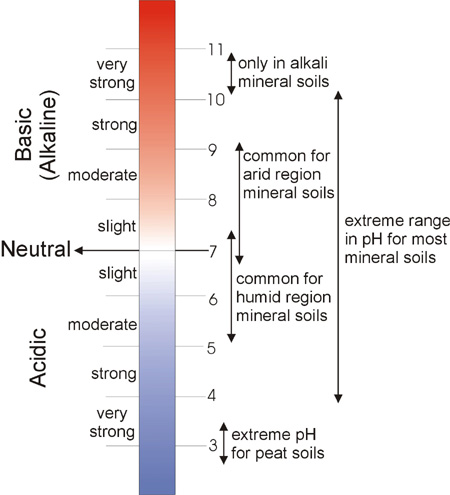
Soil pH is considered one of the key soil properties as it controls many chemical processes, such as plant nutrient availability, by controlling the chemical forms of the nutrient. The optimum pH range for most plants is between 5.5 and 7.0; however, many plants have adapted to thrive at pH values outside this range.
Common pH Ranges in Soils
Sources of Soil Acidity
Acidity in soils comes from H+ and Al3+ ions in the soil solution and adsorbed to soil colloidal surfaces.
Aluminum (Al3+) ions are released into soil solution during mineral weathering. Al3+ ions react with water (i.e., they hydrolyze) and form H+ ions. For this reason Al3+ and H+ together are considered acid forming cations.

Many processes contribute to the formation of acid soils including rainfall, fertilizer use, plant root activity and the weathering of primary and secondary soil minerals. Acid soils can also be caused by pollutants such as acid rain and mine spoilings.
- Rainfall: Acid soils are most often found in humid areas, where excess rainfall leaches base cations from the soil, increasing the percentage of Al3+ and H+ relative to other cations. Additionally, rainwater has a slightly acidic pH of 5.7 due to a reaction with carbon dioxide in the atmosphere that forms carbonic acid.
- Fertilizer use: Ammonium (NH4+) fertilizers react in the soil in a process called nitrification to form nitrate (NO3−), and in the process release H+ ions.
- Plant root activity: Plants take up nutrients in the form of ions (e.g., NO3−, NH4+, Ca2+, H2PO4−), and often, they take up more cations than anions. However, plants must maintain a neutral charge in their roots. To compensate for the extra positive charge, plants will release H+ ions from the root. Some plants will also exude organic acids into the soil to acidify the zone around their roots to help solubilize metal nutrients that are insoluble at neutral pH, such as iron (Fe).
- Weathering of minerals: Both primary and secondary minerals that compose soil contain aluminum. As these minerals weather, some ions (e.g., Ca2+, Mg2+, and K+) are taken up by plants, while others (e.g., Si4+) are leached from the soil, but due to chemical properties, iron and aluminum ions remain in the soil profile, contributing to soil acidity.
- Acid Rain: When atmospheric water reacts with sulfur and nitrogen compounds that were released through various industrial processes, the result can be the formation of sulfuric and nitric acid in rainwater.
- Mine Spoil: Severely acidic conditions can form in soils near mine spoils due to the oxidation of pyrite (FeS2).
- Potential acid sulfate soils naturally formed in waterlogged coastal environments can become highly acidic when drained or excavated.
- Decomposition of organic matter by microorganisms releases carbon dioxide, which when mixed with soil water, can form carbonic acid (H2CO3).
Sources of Basicity
Basic (or alkaline) soils have a high saturation of base cations (K+, Ca2+, Mg2+ and Na+). Soils present in regions with limestone near the surface are alkaline from the calcium carbonate (CaCO3) in limestone constantly mixing with the soil solution.
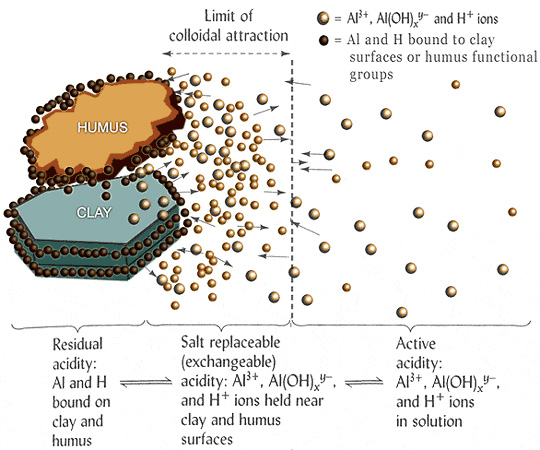
Types of Soil Acidity
Soil acidity is divided into:
- Residual acidity - refers to bound H+ and Al3+ ions that cannot be replaced by an unbuffered salt solution.
- Exchangeable (or salt-replaceable) acidity - portion of H+ and Al3+ ions that are adsorbed by soil colloids (e.g., clay and organic matter) that can be replaced by an unbuffered salt solution such as KCl or NaCl.
- Active acidity - refers to H+ and Al3+ ions in the soil solution.