LFS:SoilWeb/Interactions Among Soil Components/Chelates
Appearance
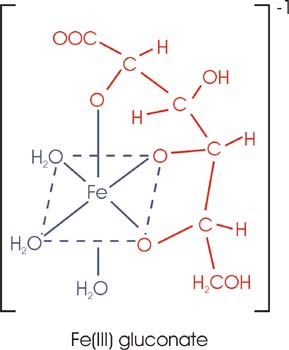
A chelate (from Greek chele = claw) is an organo-metal complex in which an organic molecule combines with a metal atom by way of two or more chemical bonds.
Organic molecules that chelate with metals include:
- Substances that are synthesized by roots
- Various humic substances that have multiple carboxyl groups
- Synthetic substances
Importance of chelates
- Dispersed and dissolved chelates readily release metal ions and contribute to nutrient metal availability. Fulvic acids form chelates which provide nutrients to plants.
- Undispersed (flocculated) and undissolved or excessively stable chelates, contribute to nutrient deficiency. Humic acids and humins form very stable chelates.
Siderophores
Under Fe-deficient conditions certain microbes and roots produce chelating ligands called siderophores. These ligands bond with Fe3+ and maintain relatively high concentrations of soluble iron.