Documentation:Soil Water Retention - Ceramic Pressure Plates
What is a Water Retention Curve?
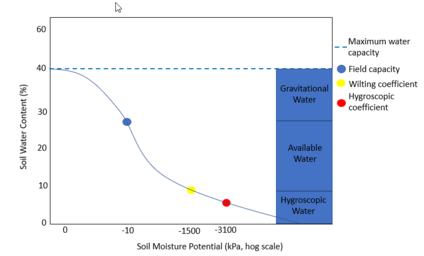
The soil water retention curve (soil moisture characteristic curve) provides a visual interpretation of the relationship between soil water content and soil water suction (Figure 1), which helps to explain the characterization of soil hydraulic properties. This relationship heavily depends on properties of soil that relate to its texture and structure (such as organic matter) (Klute, et al., 1986). The soil water characteristic is a basic soil property that is required for the study of plant-available water, infiltration, drainage, hydraulic conductivity, irrigation, water stress on the plants, and solute movement in subsurface soil (Kern, 1995).
1. Soil Saturation: It is also known as maximum water holding capacity or maximum retentive capacity. It is the amount of moisture in a soil when its pore spaces, both micro and macro-capillary, are completely filled with water. It is a rough measure of total pore space of soil. Soil moisture tension is very low between 0.01 – 0.001 kPa (1/100th to 1/1000th of an atmosphere or pF 1 to 0).
2. Gravitational Water: is free water moving through soil by the force of gravity. It is largely found in the macropores of soil and very little gravitational water is available to plants as it drains rapidly down the water table in all except the most compact of soils. This determined by the difference in water at saturation and at field capacity.
3. Field Capacity: It is the capacity of the soil to retain water against the downward pull of the force of gravity. At this stage, only micropores or capillary pores are filled with water and plants absorb water for their use. Field capacity can be measured at -10 or -33 kPa (0.1 – 0.33 atmospheres), depending on the soil characteristics. Field capacity for sandy or coarse textured soils is typically determined operationally at -10 kPa. Water at field capacity is readily available to plants and microorganisms.
4. Available Water: The amount of water required to apply to a soil at the wilting point to reach the field capacity is called the "available" water. The water supplying power of soils is related to the amount of available water a soil can hold. The available water is the difference in the amount of water at Field Capacity (-10 to -33 kPa) and the amount of water at the Permanent Wilting Point (-1500 kPa).
5. Wilting Point or Coefficient: The stage at which plants start wilting for want of water is termed the Wilting Point and the percentage amount of water held by the soil at this stage is known as the Wilting Coefficient. It represents the point at which the soil is unable to supply water to the plant. Water at wilting coefficient is held with a force of 15 atmospheres (or -1500 KPa).
6. Hygroscopic Coefficient: The hygroscopic (meaning – the maximum amount of water absorbed from the atmosphere by 100g of dry soil) coefficient is the maximum amount of hygroscopic water absorbed by 100 g of dry soil under standard conditions of humidity (50% relative humidity) and temperature (15°C). This tension is equal to a force of 31 atmospheres (or -3100 KPa). Water at this tension is not available to plant but may be available to microorganisms.
Table 1. Summary of soil moisture constants, type of water, and the force with which it is held.
| Soil Moisture Constants | Symbol | Type of Water | Force Held (kPa) | |
| Soil Saturation | Ѳsat | Gravitational Water | -0.01 to -0.001 | |
| Field Capacity | Ѳfc | Available Water | -10 to -33 | |
| Wilting Point | Ѳwp | -1500 | ||
| Hygroscopic Coefficient | Ѳhc | Hygroscopic Water | -3100 |
How the Pressure Plate Extractor Works
Pressure extractors are used in determining the water holding characteristics of soil samples. Soil samples that have been prepared to a certain bulk density or taken in situ are full saturated, and placed in the extractor with a known amount of pressure applied. This pressure is typically applied in units of kilopascals (kPa), but can be done using units of atmospheres (atm), bars, pounds per square inch (psi), or pF units (which is the log of soil tension in hectopascals (hPa)) (Appendix 3). This forces the removal of any water held in the soil at the pressure applied. By analyzing the sample at several different pressures, we can determine a moisture retention curve by plotting the known characteristic pressure versus the water content.
The pressure plate extractor is only able to measure the desaturation curve of the soil moisture characteristic (‘wet-end’). This is due to the effects of hysteresis, which is the difference in the relationship between the water content of the soil and the corresponding water potential obtained under wetting and drying processes. This means that water content in the drying (or drainage) branch of water potential – water content relationship – is larger than water content in the wetting branch for the same value of water potential.
This difference between the water content in wetting and drying processes is believed to be caused by:
(i) Irregularities in the cross-sections of the void passages or the “ink-bottle” effect (Haines 1930).
(ii) The contact angle being greater in an advancing meniscus than in a receding meniscus.
(iii) Entrapped air, which has a different volume when the soil suction is increasing or decreasing.
(iv) Thixotropic regain or aging due to the wetting and drying history of the soil (Klausner, 1991).
The hysteretic nature of the SWCC has been known for a long time but in many routine engineering and agriculture applications the SWCCs are often assumed to be nonhysteretic since the measurement of a complete set of hysteretic SWCCs is extremely time consuming and costly, and it has been difficult to represent these curves in a simple mathematical form for use in analyses (Pham, 2005).
For more measurements of the soil moisture curve at the “dry-end” there are methods such as the UMS Hyprop (Decagon Devices (Pullman, WA, USA) and UMS AG (Munich, Germany)) or the WP4C Dewpoint Potentiometer (WP4, Decagon Devices, Inc., Pullman, WA).
Modeling the Soil Water Characteristic
The shape of the water retention curves can be characterized by several models. One of the most commonly used models was created by van Genuchten (1980), and can be described as follows:
θΨ = θr + (θs - θr)/((1 + (α|Ψ|)n)1-1/n)
Where:
θΨ the water content at matric potential Ψ, (L3L-3)
|Ψ| suction pressure (L or cm of water)
θr the residual water content which is usually fitted to measured data (L3L-3)
θs the water content at saturation which is usually not fitted but taken as the measured total porosity (i.e. the water content at a matric potential of 0) (L3L-3)
α related to the inverse of the air entry suction, α > 0 (L-1 or cm-1)
ɳ a measure of the pore size distribution, ɳ > 0 (dimensionless)
This model is built for a unimodal soil pore size distribution, resulting in the standard sigmoidal S curve shape of the soil water retention curve. The accuracy of the estimated parameters relies on the quality of the acquired dataset you receive from your measurements.
In some cases, the soil water retention curve will have a bimodal distribution of soil pores. This is generally thought to be due to the presence of both macropores and micropores in the soil. A bimodal grain size distribution is typically present when there is a bimodal soil water retention curve; however, that is not always the case (Zou and Leong, 2017). When a bimodal distribution of pores exist, it is important to use soil water retention equations established for these bimodal curves. In rare cases, there may even be a trimodal distribution which uses a different soil water retention curve equation.
Materials
Field Sampling
1. Masking tape
2. Metal cores/rings
3. Bulk density sampler/ hammer or mallet
4. Piece of wood (2” x 4”) 8 inches long (approximately)
5. Plastic bags
6. Shovel
7. Hand trowel
8. Ruler
9. Buckets
10. Bubble wrap or foam
11. Butter knives
Lab Analysis
1. Saturated soil samples, either in-situ or prepared with a known bulk density
2. 5 bar Ceramic pressure plate extractor (Soil Moisture Equipment Corp., 2008)
3. 2-4 Ceramic pressure plates at 1,3,5 bar (depending on pressure being applied)
4. Wash bottles filled with deionized water (DI)
5. Outflow tubes
6. Fine and Coarse Regulator
7. Analytical balance/scale
8. 2-4 large beakers for water outflow
9. Plastic tubing
10. Tube clamps
11. Foam
12. Several large plastic trays
13. Rubber bands
14. Cotton towelettes or cheese cloth
15. Spatula
16. Sharpies
17. Drying oven
18. Oven mitts
19. Silica sand, Kaolin, or diatomaceous earth (slurry of fine particulate matter)
20. Electrical tape
21. Duct tape
22. Plastic syringe
Setup and Operation
Sample Collection and Preparation
In-situ soil sampling
1. Sampling in the rain presents issues with taking samples, such as compaction and loss of structure. It is recommended to wait until soil is relatively dry and to not sample where there is standing water present.
2. In the field, remove debris from the top of the soil, including organic matter, litter, and plant materials.
3. Depending on your sampling protocol, remove soil so that the core sits on the uppermost portion of soil you want to samples (for example: 0-4cm - place the core on the soil surface, or 5-9cm - dig into the soil and create a level surface to sample from at 5cm depth). Use the hand trowel or the shovel for digging depending on the depth and difficulty of sampling, and a ruler to measure the depth of core placement.
4. Use the bulk density sampler to pound metal cores into the soil OR use the piece of wood and the hammer (or mallet) to pound the metal cores into the soil. Do this slowly to prevent compacting the soil or disturbing the soil structure.
5. Once the metal ring is fully inserted into the soil, you can use the shovel or hand trowel to excavate the core. Use a butter knife to level the exposed edges of the cores.
6. Place the cores into the bottom of the bag, tight against the plastic, and wrap the core in plastic, masking tape, and bubble wrap to prevent accidental compaction and damage of soil structure.
7. Store intact cores in the fridge at approximately 4°C to limit the growth of algae and fungi on the cores prior to analysis.
72 hours-1 week prior to set-up
1. Record the weight of a square of cheesecloth and rubber band to be used for a particular core and place the cheesecloth and rubber band on the bottom of your soil core (the side that would be lower in the soil profile).

2. Fill a small tub with water to a about ¾ of the depth of your sampling core.
3. Place the soil sample into the water with the bottom facing down.
4. Allow cores to fully saturate for a period of 1-2 days for sandy or silty soils, and up to 3-7 days for clayey soils. Soils are fully saturated when the top surface is ‘glistening’ (Figure 2).
5. Prepare one empty sampling ring with a cheesecloth and rubber band to be used as blank, and wet this core similarly.
6. Only if there is an uneven or bumpy layer of soil that will be placed onto the plate, otherwise there is no need to use diatomaceous earth. Weigh the soil core while it is fully saturated, remove the rubber band and cheesecloth to apply a fine layer (1-3mm) of diatomaceous earth paste (or kaolin or silica slurry) on the bottom of the core, re affix the rubber band and cheesecloth to the ring, and place the saturated sample onto the pressure plate.
7. Weigh the sample again to estimate the amount of diatomaceous earth past added to the sample.
Ex-situ soil sampling
1. In the field, take at least one core sample as directed above in steps 1-4.
2. Place this sample into a separate bag and use it to calculate the bulk density of the soil at this sampling point. Follow procedures for determining soil bulk density. Place your core samples in a 105°C oven for 24hrs and weigh it to determine the oven-dried of your sample.
3. In addition, collect about 1kg of soil in a bag, which will be used to prepare your actual samples for the pressure plates.
72 hours - One week prior to set up
1. Fill a beaker with approximately 100g of your soil sample.
2. Saturate the sample and allow to the water to permeate the cores for 1-2 days for sandy or silty samples, and 3-7 days for clay texture soils.
3. Calculate the weight of your soil sample at saturated water content for the volume of the rings you will be using.
4. Weigh your rings and place a wet cheesecloth around each ring with a rubber band and weigh again.
5. Place into the ring the amount of soil determined above.
6. Record which soil sample went into the corresponding soil core, using tape if there are a large amount of samples.
7. Prepare one empty sampling ring with a cheesecloth and rubber band to be used as blank, and wet this core similarly.
8. Place prepared sample onto your saturated pressure plate.
24-48 Hours prior to set-up
Preparing the plates

1. Locate the ceramic plates that you will be using for your protocol. Up to 4 plates can be used at a time, and approximately 12 samples per plate (48 samples total). It is recommended to wet excess plates, as there may be a chance that one of the plates is not working properly.
2. Wear nitrile gloves when handling ceramic plates to prevent accumulation of oil and soil on the plates which can lead to clogging of porous ceramic material and reduction of connectivity between soil cores and ceramic surface.
3. Ensure that all plates are in good working condition – no holes in the bottom of the plate, all neoprene diaphragms are pointed up and can keep approximately 1cm of water on the ceramic plate. Use electrical tape or duct tape when appropriate to repair/prepare the plates.
4. Use approximately 500ml of water to create a 1cm layer of water above the ceramic plate. Visually inspect to make sure no water is leaking off of the plate or through tears in the rubber (Figure 3).
5. Allow plates to sit with water on top for a minimum of 24hrs to ensure that they become fully saturated.
Pressure Plate Operation
1. Prior to set up of pressure plates, familiarize yourself with the manifold set-up (Figure 4).

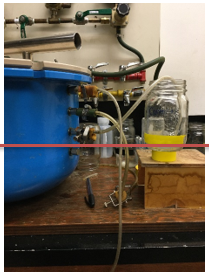
2. Complete a test run of the empty plates to check for air leaks prior to placing cores on the plates.
3. With the chambers open, make sure that the outflow tubes are sitting in jars of water, and that the water level in the jar lines up with the level of the pressure plate inside the chamber (Figure 5), otherwise you will create a difference in the pressure gradient and either force water into your soil, or force water out. Mark the level of water with a piece of tape.
Figure 5. Make sure that the water level in the jar you are using to collect water is parallel with the bottom of the pressure plate in the chamber.
4. Place the extractor lid on top of the chamber and fasten the screws with the wingnuts on top. Ensure that the square bottom sits in the groove of the extractor. Fasten them one at a time, and tighten the opposite wingnut from the one you start on as you go around the lid.
5. After all the wingnuts are in place, make sure to tighten them once more until all 6 are secure.
6. Follow steps ‘Applying Air Pressure’
7. Check for leaks in the pressure plates by examining the tubes for air bubbles.
8. Once you are satisfied there are no leaks follow steps for ‘Releasing Air Pressure’
9. After you have prepared your samples, you can add a small amount of additional water to the top of each core, to ensure that they are fully saturated. Spray 1mm of water across the plate to ensure hydraulic contact with the soil ring samples and plate. If you have excess water on the plate, remove it using a plastic syringe.
10. Place the prepared samples and pressure plates inside the pressure chambers.
11. Ensure that you have attached the outflow tube inside the chamber to the outflow port on the ceramic pressure plate (Figure 6).
12. Repeat steps 2-4 for each of the plates that you will be using.
13. Place the extractor lid on top of the chamber and fasten the screws with the wingnuts on top. Ensure that the square bottom sits in the groove of the extractor. Fasten them one at a time, and tighten the opposite wingnut from the one you start on as you go around the lid.

14. After all the wingnuts are in place, make sure to tighten them once more until all 6 are secure.
Applying Air Pressure
1. For the main valves, a horizontal position indicates they are in the open position, while for the release and exhaust valves, pointing toward you indicates that they are in the open position. For the air sources and regulators, turn counterclockwise to release pressure and clockwise load pressure.
2. If you intend on using the pressure plates at the same pressure, it is recommended that you attach them to the same coarse regulator. However, if you want to run them differently, the left coarse regulator operates from 0-100 psi (0-690 kPa), and the right regulator operates from 0-15 psi (0-100 kPa).
3. Make sure that the coarse and fine regulators are open for the system you are using, as well as the main valve and at least one exhaust valve are all open. Make sure the release valve is closed, in order to prevent accidental pressure load to the plates.

4. Open the pressurized air release valve (Figure 7) to make sure air is flowing.
5. Close the exhaust valves.
6. Turn the coarse regulator clockwise several times to load the pressure onto line and then turn the fine regulator clockwise to set the approximate desired pressure. To prevent an air leak, minimize the pressure difference between the coarse and fine regulators. Observe the pressure gauge and adjust the fine regulator to the target pressure of 0.10 bar (= 100 hPa).
7. Open the release valve to let air pressure reach the plates.
8. Water should flow through and collect in the jars.
9. You will need to increase / decrease the water level in the jar to the level marked by the tape. This will maintain the correct pressure head. As water is pulled from the cores/plates the water level in the jar will increase and evaporation will decrease the water level for longer run times.
10. Adjust the water heights twice a day to the taped level, once at the beginning of the day, and once at the end.
11. Once the water flow into the jars stops for approximately 24 hours, the soil samples inside have reached the equilibrium water content at the applied pressure. This should take approximately 48-72 hours for coarse and medium textured soils, and 120 hours for fine textured and organic soils.
12. Upon equilibrium, close off the outlet tube to ensure that no water re-enters the plate when the pressure is reduced.
13. For samples that take longer to equilibrate, and for 15 bar samples, make sure to check on the compressor at least 2-3 times per week. Ensure that the oil level is optimal, there are no oil leaks, no air leaks, and no burnt smells.
Releasing Air Pressure
1. Close the pressurized air valve (Figure 7).
2. Open up one of the exhaust valves.
3. Wait for the fine and coarse regulators to reach zero.
4. Loosen all the wingnuts from the extractor lid; only remove the lid once the pressure has been released.
5. Remove the lid, and set it down on the bar side so that you do not damage the sealing area.
6. Unfasten the outflow tube from the pressure plate.
Weighing Samples
1. Tare the weight of a spatula, and use it to pick up the soil sample pick up the sample ring from the plate by slightly twisting to break their contact from the ceramic top.
2. Weigh the total weight of the ring confined samples, including the rubber band and cheesecloth with the spatula.
3. Only if diatomaceous earth is being used:
a. Gently remove the rubber band and cheesecloth and weigh. Remove any attached soil from the cheesecloth and rubber band using a brush or pick.
b. Use a small brush to remove as much diatomaceous earth (silica or kaolin) (as necessary) and weigh the sample again.
c. Ensure that any soil attached to the cheesecloth and rubber band are weighed with the soil.
d. After weighing the samples, replace the diatomaceous earth (silica or kaolin), the rubber band, and the cheesecloth. Place the samples into a covered bin to prevent evaporation of water from the samples.
4. Weigh the samples on the second pressure plate, and repeat steps 1-2.
5. Apply water to the pressure plate to create hydraulic continuity between the plate and the ring samples and place the weighed ring samples onto the ceramic plate. Avoid spraying any water onto the ring samples.
6. The samples are then ready for the second pressure application.
7. This process can be repeated for the 10 kPa ( 0.1 bar), 33 kPa (0.33 bar), and 100 kPa (1 bar (or 0.9) bar readings on the 1 bar pressure plates. Complete For the 300 kPa 2 and (3 bar) pressure readings on the 3 bar pressure plates, and 500 kPa4 and (5 bar ) readings on the 5 bar pressure plates, and 1500 kPa at 10 and (15 bar) readings on the 15 bar pressure plates .
8. Ideally, additional measurements can be made depending on time and budget constraints. If your samples are showing signs of having a bimodal soil moisture characteristic, additional reading may be needed.
9. Transfer the soil samples to metal drying tins and place into a drying oven at 105°C for 24 hrs or until a constant weight is obtained.
10. Use oven mitts to transfer samples from the oven into desiccators unless weighing immediately to obtain the oven-dry soil weight.
11. Place the soil sample into the original plastic bag to store for future analyses.
Calculate the soil moisture content of the samples on a volumetric basis at each applied pressure using the following formula:
θ = ((Ws (g) - Wd (g)) / Pw (g cm-3))/Vs (cm3)
Where;
Ws = Weight of soil at each applied pressure (g)
Wd = Weight of oven dry soil (g)
Pw = Density of water in lab conditions (~1g cm-3)
Vs = Volume of soil in the ring (cm3)
Calculate the oven-dry bulk density:
Db (g cm-3) = Wd (g) / Vs (cm3)
Where;
Wd = Weight of oven dry soil (g)
Vs = Volume of soil in the ring (cm3)
Calculate the porosity (This can be compared to the saturated soil weight, as typically the will be quite close, which gives you an opportunity to double check your measurements):
Porosity (cm3/cm3) = (Ps-Pb)/Ps = 1- (Pb/Ps)
Where;
Ps = Density of solids (approximately 2.65g cm-3)
Pb = Bulk density (g/cm3)
Cleaning the Ceramic Plate Extractor
- After conducting the final pressure setting, remove the outflow tube from the outflow port of the pressure vessel. With both hands, lift the top and bottom ceramic plates by plate. Avoid using soap when scrubbing and rinsing the ceramic plates with DI water. Do not press fingers on the ceramic part of the plate to prevent plugging the ceramic plate pores with soil and oil. Let the ceramic plate air-dry in a secure place before storing it.
- Wipe the pressure plate extractor with a paper towel and air-dry the inside of the extractor to prevent the steel clips and inner walls from rusting. After drying, close the extractor with its lid secured lightly by a single wingnut. Ensure that the main valve on the pressurized air is closed.
Data Management and Recording
Data Recording
- It is recommended to repeat the pressure plate measurements a minimum of 2 (or 3, time permitting) times per sample at each pressure reading.
- It is not recommended to reuse ex-situ samples between different pressure plate capacities (1 bar, 3 bar, 5 bar, and 15 bar).
- Use the attached data recording sheet as a template, and modify the pressure readings to those you are interested in, as well as the number of replicates per sample you are interested in.
Analysis of Data
Soil Water Characteristic Determination
Soil moisture values for saturation, field capacity, permanent wilting point, and available water content should be determined through statistical analysis. It is important to obtain good measurements of soil moisture at the soil moisture constants to use as a comparison to other soil properties such as texture and structure.
Troubleshooting
- For any problems with the pressure plate extractor, refer to its operating instructions at https://www.soilmoisture.com/pdfs/Resource_Instructions_0898-1600_1600%205%20Bar%20Pressure%20Plate%20Extractor.pdf
- Ensure that there are no leaks in the manifold by using a combination of dish soap and water to make a solution which will bubble when applied to the manifold. Unscrew the leaky area, clean the area thoroughly with a brush or steel wool to remove the old silicone tape, and replace the silicone tape before reattaching. Usually enough tape to go around the pipe circumference with a small 1/2cm over lap is enough. Put the silicone tape on in a counterclockwise fashion so that when you tighten the pipe in a clockwise fashion, the tape does not bunch up.
- Try replacing an outflow tube and tightening the tube against the ouflow port using a piece of sponge and hose clamps.
- http://www.worldagroforestry.org/sites/default/files/METH05V01%20Soil%20Moisture.pdf
- If the plates are looking grimy and will not transfer water properly (i.e.: plate readings are quite different from other plates and samples seem to have variable levels of water) you can try to wash the plates using a small amount of dawn dish soap and an enfant's toothbrush to scrub the plate (an enfant’s toothbrush has much softer bristles than an adults brush).
- If there are any concerns regarding the system set up, you can contact Lewis Fausak (the applied biology research and education technician) at lewis.fausak@ubc.ca or lkfausak@gmail.com.
References
1. Brady, Nyle C, and Ray R. Weil. 2002. The Nature and Properties of Soils. Upper Saddle River, N.J: Prentice Hall, 2002.
2. Brooks, R. H. and Corey, A. T. 1964. Hydraulic properties of porous media, Hydrol. Paper 3, Colorado State Univ., Fort Collins, CO, USA. 409, 410
3. Brutsaert, W. 1966. Probability laws for pore-size distribution, Soil Sci., 101, 85–92. 412
4. Campbell, G. S. 1974. A simple method for determining unsaturated conductivity from moisture 25 retention data, Soil Sci., 117(6), 311–317. 409, 410
5. Durner, W. 1994. Hydraulic conductivity estimation for soils with heterogeneous pore structure, Water Resour. Res., 30(2), 211–223. 409, 410, 413, 422
6. Fong, A., A. Tuli and J. Gonzales. 2018. Measuring Saturated Hydraulic Conductivity with Constant Water Head Using an Eijkelkamp Laboratory-Permeameter. Standard Operating Procedure Number: METH015.00. California Department of Pesticide Regulation, Environmental Monitoring Branch, Sacramento, CA.
7. Haines, W.B. 1930. Studies in the physical properties of soil – V: The hysteresis effect in capillary properties and the modes of water distribution associated therewith. Journal of Agricultural Science, 20: 97–116.
8. Kern, A. 1995. Evaluation of soil water retention models based on basic soil physical properties. Soil Sci. Soc. Am. J. 59: 1134-1141.
9. Klausner, Y. 1991. Fundamentals of continuum mechanics of soils. Springer-Verlag, New York.
10. Klute, A. 1986. Water Retention: Laboratory Methods. Ed. A. Klute, Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods, 2nd ed., American Society of Agronomy, Madison, Wisconsin, U.S.A.
11. Kosugi, K. 1994. Three-parameter lognormal distribution model for soil water retention, Water Resour. Res. 30(4), 891–901, 1994. 420 5
12. Kosugi, K. 1996 Lognormal distribution model for unsaturated soil hydraulic properties, Water Resour. Res. 32(9), 2697–2703, 1996. 409, 410, 411, 420
13. Marquardt, D.: An algorithm for least-squares estimation of nonlinear parameters, J. Soc. Indust. Appl. Math., 11, 431–441, 1963. 417
14. Mualem, Y. A.: A new model for predicting the hydraulic conductivity of unsaturated porous 10 media, Water Resour. Res., 12, 513–522, 1976. 410
15. Nemes, A., Shaap, M. G., Leij, F. J., and Wosten, J. H. M.: Description of the unsaturated soil ¨ hydraulic database UNSODA version 2.0, J. Hydrol. (Amsterdam), 251, 151–162, 2001. 411, 418, 419
16. Pham, Hung & Fredlund, Delwyn & Barbour, Sidney. (2005). A Study of hysteresis models for soil-water characteristic curves. Canadian Geotechnical Journal - CAN GEOTECH J. 42. 1548-1568. 10.1139/t05-071.
17. Poulsen, T. G., Moldrup, P., Iversen, B. V., and Jacobsen, O. H.: Three-region Campbell model 15 for unsaturated hydraulic conductivity in undisturbed soils, Soil Sci. Soc. Am. J., 66, 744–752, 2002. 409
18. Reynolds, W. and Topp, G. 2006. Water desorption and imbibition: tension and pressure techniques. Soil sampling and methods of analysis. Canadian Society of Soil Science. Taylor and Francis Group.
19. Segawa, R. 2003. Personnel Organization and Responsibilities for Studies. Administrative Standard Operating Procedure Number: ADMN002.01. California Department of Pesticide Regulation, Sacramento, CA. https://www.cdpr.ca.gov/docs/emon/pubs/sops/admn0201.pdf
20. Seki, k. 2007. SWRC fit – a nonlinear fitting program with a water retention curve for soils having unimodal and bimodal pore structure. Hydrol. Earth Syst. Sci. Discuss., 4, 407–437, 2007. Retrieved from https://www.hydrol-earth-syst-sci-discuss.net/4/407/2007/hessd-4-407-2007.pdf
21. Seki, K.: A program for nonlinear fitting of soil water retention curve written in numerical calculation language GNU Octave, J. Jpn. Soc. Soil Phys. (in Japanese), in press, 2007.
22. Soil Moisture Equipment Corp. 2008. Operating Instructions for 1600 5 Bar Ceramic Plate Extractor. Santa Barbara, CA. https://www.soilmoisture.com/pdfs/Resource_Instructions_0898- 1600_1600%205%20Bar%20Pressure%20Plate%20Extractor.pdf
23. Stephens, D. 1996. Vadose Zone Hydrology. Boca Raton: CRC Press, https://doi.org/10.1201/9780203734490
24. Taylor, S.A. and G.L. Ashcroft. 1972. Physical Edaphology. The Physics of Irrigated and Nonirrigated Soils. W.H. Freeman and Company, San Francisco, CA.
25. Teixeira, Wenceslau & Sinclair, Fergus & Huwe, Bernd & Schroth, Götz. (2003). Soil water.
26. Tuli, A. 2015. Procedure for determining soil particle density using Gay-Lussac specific-gravity bottles. Standard Operating Procedure Number: METH012.00. California Department of Pesticide Regulation, Environmental Monitoring Branch, Sacramento, CA.
27. van Genuchten, M. T.: A closed-form equation for predicting the hydraulic conductivity of unsaturated soils, Soil Sci. Soc. Am. J., 44, 892–898, 1980. 409, 410
28. van Genuchten, M. T., Leij, F. J., and Yates, S. R.: The RETC code for quantifying the hydraulic functions of unsaturated soils, Report No. EPA/600/2-91/065, R. S. Kerr Environ. Res. Laboratory, U.S. Environmental Protection Agency, Ada, Oklahoma, 1991. 409 25
29. Vogel, T., van Genuchten, M. T., and Cislerova, M.: Effect of the shape of the soil hydraulic functions near satur
30. Zou, L. and E.C. Leong. 2017. Soil with Bimodal Soil-Water Characteristic Curve. PanAm Unsaturated Soils. Fundamentals. GSP 301, 2018
Appendix
Table A1. Pressure conversion table
Pressure Conversion Table
| Bar | kPa | Psi | pF |
| 1/10 | 10 | 1.45 | 2.0000 |
| 1/3 | 33 | 4.35 | 2.5185 |
| 1 | 100 | 14.5 | 3.0000 |
| 3 | 300 | 43.5 | 3.4771 |
| 5 | 500 | 72.5 | 3.6990 |
| 15 | 1500 | 217.6 | 4.1761 |
Table A2. Data Collection Sheet
| Sample Number | Ring Number | Bar | Psi | cm water column (potential in kPa) | Weight | V = Volume of core ring = __________cm3 | ||||||
| Wet weight (sample, ring, cloth, elastic) | Dry weight (Sample, ring, cloth, elastic) | Weight of ring, cloth, elastic | Weight of soil water | Weight of dry soil | Gravimetric water content | Bulk Density | Volumetric water content | |||||
| A | B | C | D=A-B | E=B-CB | W=D/E | Db=E/V | Ѳ=W*Db | |||||
| 0.1 | 1.45 | 10 | ||||||||||
| 0.33 | 4.35 | 33 | ||||||||||
| 1 | 14.5 | 100 | ||||||||||
| 3 | 43.5 | 300 | ||||||||||
| 5 | 72.5 | 500 | ||||||||||
| 15 | 218 | 1500 | ||||||||||
Table A3. Unit Conversion for different pressure units.
| 1 bar | 1 kilopascal (kPa) | 1 atmosphere (atm) | 1 pound per square inch (psi) | |
| 1 bar | 1 | 100 | 0.986923 | 14.5038 |
| 1 kilopascal (kPa) | 0.01 | 1 | 0.00986923 | 0.145038 |
| 1 atmosphere (atm) | 1.01 | 101.325 | 1 | 14.6959 |
| 1 pound per square inch (psi) | 0.07 | 6.89476 | 0.068046 | 1 |