Documentation:CHBE Exam Wiki/5.8 - Path and State Functions
5.8 – Path and State functions
5.8.0 – Learning Objectives
By the end of this section you should be able to:
- Build further knowledge of state variable and state functions.
- Explain the difference between state and path functions.
- Classify thermodynamic terms as state or path variables.
5.8.1 – Introduction
State and path functions are how variables in a system change from an initial state to a final state. In this notebook, you will learn about state and path variables, state and path functions, their differences, and their uses.
5.8.2 – State Functions
Remember that guy who went up Mount Everest? Imagine you are a tourist at the bottom and see that guy on top of the mountain. Regardless of how he got there, that guy got there and is 8,848m higher than you are and thus he has 8679888J of potential energy more than you (assuming they weigh 100kg).
A State function is the change of the state of a variable regardless of the path it takes. Internal Energy , Enthalpy , Pressure , Temperature , and Volume are state functions.
But how did that guy get up there? Did he skydive out of a plane? Did he climb up there himself? Was he carried up by a Sherpa? Now we are concerned about the path the guy took to get up there.
A Path function is the transition of path variables from one state to another. Work and Heat are path variables. They change depending on what path is chosen to reach a state.
5.8.3 – Path functions change the amount of work done on/by a system:
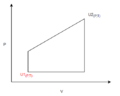
Let’s take a look at the diagram below:
is at an initial state of and . Our goal is to get to . Here we are presented with 2 options.
In Path 1, the system is isochorically compressed and then expanded to the state U2. In Path 2, the system is isobarically expanded, then isochorically compressed to the state U2.
As Work is , it is clear that the work done is much greater in the first path. This is because the magnitude of volumetric expansion was executed at a higher pressure than the second path.
Furthermore: We can infer that there is more heat transferred to system 1 since U2 is constant for both and using the information from the first law , if W is larger, then Q must be larger to raise the value of U2