Documentation:CHBE Exam Wiki/5.6 - Questions on Cv and Cp
5.6 – Questions on Heat Capacity
5.6.0 – Learning Objectives
By the end of this section you should be able to:
- Visualize how and are used to calculate and solve various problems.
5.6.1 – Introduction
This notebook will cover questions relating to the and .
5.6.2 – Question 1:
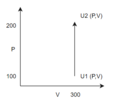
Argon is in a closed system at P = 100 atm, V = 300 L, and T = 600 K is brought to a state at P = 200 atm, volume at 300 L and 900 K. What is the change of internal energy in the system?
1. Draw a diagram of the process taking place and write the relevant equation:
2. Add simplifications and assumptions
- We assume the only work done is via but since there is no change in volume and the equation is reduced to .
- Since we are working with a closed system, internal energy can be substituted as
- Since we are working with argon which is an ideal monatomic gas, we can use the ideal gas law to fairly accurately estimate the number of moles and use as where
note: watch out for units sometimes you will get and Pa!
3. Solve equations
- Since we are given a direct relationship between and we do not need to know any formulas for the heat.
5.6.3 – Question 2:
18 kg of ice stored at -22 C is melted then brought to 32 C. How much energy is transferred in this system?
Assume heat capacity is a constant value. The specific heat capacity of ice is 2.108 ; the latent heat of fusion is -334 and the specific heat capacity of water is 4.184 .
1. Draw the diagram of the process taking place and write down the relevant equations.
2. List simplifications and assumptions
- Any specific heat capacity used here is assumed to be constant because no information suggests otherwise. This is often the case
- The mass is constant in the system.
- Since all units are in Celsius and heat is in terms of a reference, we are not concerned with converting to Kelvin.
3. Solve equations. Mind the units
(by the same unit conversion of ice).
5.6.4 – Question 3:
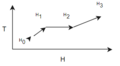
n-butane is brought from 450 C to 923C. Using the formal definition and formula of heat capacities, find the heat required to bring n-butane to this temperature.
Preamble: Because the temperature range that we are measuring is extremely large, it is important to capture the true heat capacity to evaluate the enthalpy. Here we must assume that the pressure is adequately low to assume ideal gas law.
- Draw the diagram of the process taking place and write down the relevant equations.
- List simplifications and assumptions
- Solve equations Note mind your units! Kelvin and Celsius matter! For us, we are told to use Celsius.
Taking the tabulated data from table B2 and expanding the enthalpy equation:
Note: table B2's powers are all supposed to be negative.