Documentation:CHBE Exam Wiki/5.2 - Heat and Work
5.2 – Heat and Work
5.2.0 – Learning Objectives
By the end of this section you should be able to:
- Understand the characteristics of heat and work.
- Understand the various forms that heat and work come in.
- Utilize the first law along with heat and work.
5.2.1 – Introduction
This notebook will cover the expanded form of the first law:
and when we are observing the change of a system the first law becomes
Where is the change. This breaks down the first law into
Heat and Work usually do not take the delta sign in front of them because their value is given as energy transferred. As the past notebook states. Energy is the cumulative terms of . Breaking these down into their respective initial and final states and plugging it into the first law we get
5.2.2 – Heat
Heat is the quantity of energy that is added or removed from the system. Clay is often fired in kilns to harden the structure of the material. This adds massive amounts of heat to the system so that the silica particles in the clay form a lattice.
If there is no heat transfer becomes 0 and the process is known as an adiabatic process.
5.2.3 – Work
Work is the mechanical energy that the system either takes or gives. The full term for is work = . Sign convention of work: The textbook defines
as work done by the system
and
as work done on the system
Example
Pumping a water gun: solving the work equation (which will be solved in later chapters) gives a negative work which is substituted into the first law equation:
Resulting in a net positive increase of internal energy assuming that is positive (more energy to decimate your opponents... or at least before you have to pump again).
an ejection seat launching a pilot away: solving the work equation, gives a positive work which is substituted into the first law equation :
Assuming little heat is gained, the internal energy of the seat is now smaller.
Example - 5.2 Heat and work
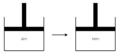
In a batch mixing reactor, there might be pressure and heat changes that might affect the total energy of the system. The following scenario is observed:
There is gas in the reactor cylinder. The initial gas temperature is 20 °C. The cylinder is placed in boiling water with the piston held in a fixed position. Heat in the amount of 2.00 kcal is transferred to the gas, which equilibrates at 100 °C. The pressure has also found to be increased at equilibrium. The piston is then released, and the gas does 100 J of work in moving the piston to its new equilibrium position. The final gas temperature is 100 °C.
Step 1
In this step, the system absorbs heat that raises its temperatures by 80 °C.
Resulting in the equation:
We still need to convert to Joules as it is the SI unit of energy.
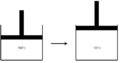
Step 2
Resulting in the equation:
Note: The work is positive because the the system is doing work on the surroundings.