4.8 – Vapour Pressure
4.8.0 – Learning Objectives
By the end of this section you should be able to:
- Understand the concept of vapour pressure.
- Use the Antoine equation.
- Know when you should use Raoult's law.
- Know when you should use Henry's law.
- Distinguish between the different types of saturation and humidities.
4.8.1 – Introduction
Vapour pressure is the pressure a vapour exerts with its solid or liquid at a given temperature at equilibrium. This means that vapour pressure is a good indication of a liquid's evaporation rate or a solid's sublimation rate.
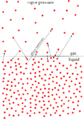
4.8.2 – Vapour Pressure
As you can see in the figure below, vapour pressure is at equilibrium. This means that there is as much vapour being produced from evaporation as vapour being consumed due to condensation. The magnitude of the vapour pressure tells you how volatile the liquid is. The higher the vapour pressure, the higher the volatility.
4.8.3 – Antoine equation
The Antoine equation is simple yet crucial equation the relates vapour pressure and temperature. The equation is:

Where A, B, and C are constants that can be found in tables and


4.8.4 – Raoult's Law and Henry's Law
Raoult's law and Henry's law provide us with a relationship between the partial pressure of a substance,  , in the gas phase and the mole fraction of the same substance,
, in the gas phase and the mole fraction of the same substance,  , in the liquid phase. Raoult's law is generally valid when
, in the liquid phase. Raoult's law is generally valid when  is close to 1, which means the liquid is almost pure A. Henry's law is generally valid when solutions of
is close to 1, which means the liquid is almost pure A. Henry's law is generally valid when solutions of  are close to 0.
are close to 0.
The expression for Raoult's law is:

where





The expression for Henry's law is:

where





4.8.5 – Saturation and Humidity
The term saturation refers to any gas-vapour system. Humidity refers specifically to an air-water system. There are four main types of saturation and humidity:
Relative Saturation (Relative Humidity):

where


Molal Saturation (Molal Humidity):

where


Absolute Saturation (Absolute Humidity):

where




Percentage Saturation (Percentage Humidity):

where



Each one of these different types of saturation and humidity’s are useful in different scenarios.
4.8.6 – Problem Statement
Problem 1
Question
Humid air at  , 1 atm, and 73 % relative humidity is fed into a unit operation at a rate of
, 1 atm, and 73 % relative humidity is fed into a unit operation at a rate of  . The vapor pressure of water at 1 atm and
. The vapor pressure of water at 1 atm and  is
is  . Determine
. Determine
a) the molar flow rates of water, dry air, and oxygen, assuming oxygen makes up 21% of dry air.
b) the molal humidity, absolute humidity, and percentage humidity of the air.
==== Answer
a) the molar flow rates of water, dry air, and oxygen, assuming oxygen makes up 21% of dry air
First, we must determine the partial pressure of the water.

Then using Raoult's Law, we can fine the vapour mole fraction of the water

Assuming the humid air behaves ideally, we can use the ideal gas law to solve for the molar flow rate of the wet air

Since we know the mole fraction of the water is

then we can solve the mole fraction of the dry hair by

Since the mole fraction of oxygen in dry air is

We can finally solve for the molar flow rate of water, dry air, and oxygen.



b) the molal humidity, absolute humidity, and percentage humidity of the air
We have already solved for the partial pressure of the water vapor so solving for the molal humidity

The MW is 18.00 g/mol while the average MW of dry air is 28.9 g/mol. With that knowledge, we can solve for absolute humidity

Finally, to find the percentage humidity we must

Problem 2
Question
Find the temperature at which the vapour pressure of water is  where
where



Answer
Using Antione’s equation

we can plug in the values and solve for  .
.