4.2 – Standards
4.2.0 – Learning Objectives
By the end of this section you should be able to:
- Understand what standard temperature and pressure (STP) is.
- Understand what standard cubic meters and feet are.
4.2.1 – Introduction
In engineering and science, it is useful to have standards to use as reference points. If most people in the field you work in use one type of standard, then confusion, mistakes, and conversions can be prevented.
4.2.2 – Standard Temperature and Pressure (STP)
Standard temperature and pressure (STP),  and
and  , are used as a reference point for many laws, including the ideal gas law. When the ideal gas law
, are used as a reference point for many laws, including the ideal gas law. When the ideal gas law

is divided by the reference point ideal gas law

you get

Since we got rid of the R, the constant is not needed anymore. The SI units for (STP) are



4.2.3 – Standard Cubic Meters and Standard Cubic Feet
Standard cubic meters (SCM), is used to denote  at STP. Standard cubic feet (SCF), is used to denote
at STP. Standard cubic feet (SCF), is used to denote  at STP. A volumetric flow rate of 21.3 SCMH is
at STP. A volumetric flow rate of 21.3 SCMH is  at
at  and 1 atm.
and 1 atm.
4.2.4 – Problem Statement
Problem 1
Question
You are a chemical engineer at the Burnaby oil refinery. Your colleague has asked you to inform him on how much gasoline is flowing through a pipe in SCFH. You measure the flow rate and find that gasoline is flowing at  at
at  The reference point for the density of gasoline is
The reference point for the density of gasoline is  at
at  . The correction factor for the gasoline at
. The correction factor for the gasoline at  is
is  . The correction factor for the gasoline at
. The correction factor for the gasoline at  is
is  . What is the flow volumetric SCFH flow rate?
. What is the flow volumetric SCFH flow rate?
Answer
First, we must find the mass flow rate at  . This is because mass must be conserved at any temperature.
. This is because mass must be conserved at any temperature.

Now that we have the mass flow rate, we can calculate the volumetric flow rate at  .
.

Finally, we can convert the volumetric flow rate to the proper units of  .
.

Problem 2
Question
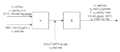
(A = Evaporator, B = Compressor)
Acetone ( ) is fed at a rate of 400 L/min into a heated chamber, where it evaporates into a nitrogen stream. The gas leaving the heater is diluted by another nitrogen stream flowing at a measured rate of 419
) is fed at a rate of 400 L/min into a heated chamber, where it evaporates into a nitrogen stream. The gas leaving the heater is diluted by another nitrogen stream flowing at a measured rate of 419  (STP)/min. The combined gases are then compressed to a total pressure
(STP)/min. The combined gases are then compressed to a total pressure  = 6.3 atm gauge at a temperature of 325 °C. The partial pressure of acetone in this stream is
= 6.3 atm gauge at a temperature of 325 °C. The partial pressure of acetone in this stream is  = 501 mm Hg. Atmospheric pressure is 763 mm Hg
= 501 mm Hg. Atmospheric pressure is 763 mm Hg
What is the volumetric flow rate of the nitrogen entering the evaporator if the temperature and pressure of this stream are 27 °C and 475 mm Hg gauge?
Solution
Since there are no assumptions to write, let us go ahead solve the question, first by changing all of the relevant units to a single uniform unit:

Note: The density of acetone was found in Table B1. of Elementary Principles of Chemical Processes.
We then should calculate the mole fractions of the exit streams, based on the partial pressures of the components.
Remember that the actual pressure of a system is gauge pressure added onto atmospheric pressure:




Let us turn our attention to  now. Based on our knowledge of standards, we now know that we can deduce the moles of
now. Based on our knowledge of standards, we now know that we can deduce the moles of  in the stream based on STP conditions, using the Ideal Gas Law:
in the stream based on STP conditions, using the Ideal Gas Law:



Now with all the necessary information, we can move onto the mass balances:








Finally, we can solve for the volume of nitrogen that is entering the system, as we can go back to the Ideal Gas Equation: