Course:MATH110/Archive/2010-2011/003/Teams/Zurich/Homework 13 Logarithmic Scale - pH
What is pH?
The mathematical definition of pH (power of Hydrogen) is the negative logarithmic value of the Hydrogen ion (H+) concentration.
The acidity or alkalinity of a substance (ph) can be written as the equation:
When is the hydrogen ion solution in moles per litre.
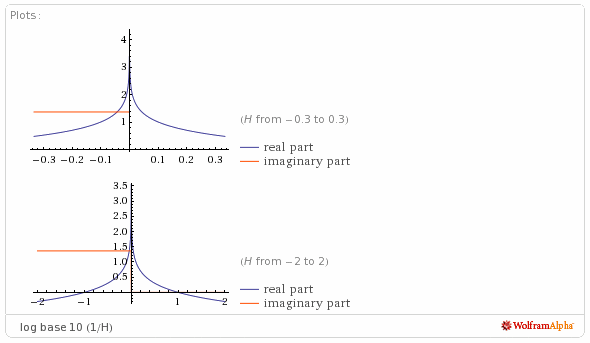
The pH graph looks like this, courtesy of WolframAlpha:
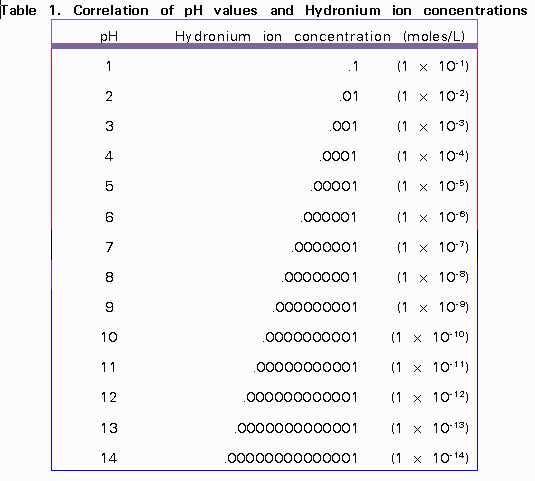
Here is a table showing the correlation of pH values and Hydronium ion concentrations:
Courtesy of http://wwwchem.csustan.edu/chem3070/3070m07.htm]
Example
Suppose we test tomato juice and we find that the is equal to 0.0001. How do we find the pH value and determine whether the tomato juice is acidic or alkaline?
1) Substitute into our logarithmic substance formula
In this case: Hence: pH = 4
This means that the tomato juice is acidic with a pH of 4.
Since the negative log of H+ is used in the pH scale, we will usually get positive values (as long as H+ is below 1). Additionally, the larger our pH, the smaller our H+.
The pH scale turns a concise scale into a large scale between the values 0 and 1.
We know that there is an infinite number of values between 0 and 1 which shows that using a log scale allows us to covert small quantities into large quantities.
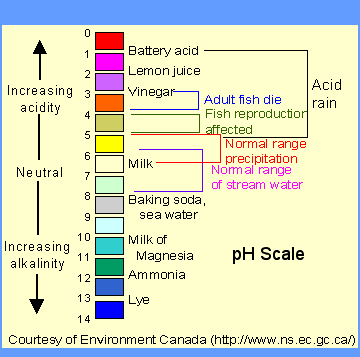
The pH scale is logarithmic and as a result, each whole pH value below 7 is ten times more acidic than the next higher value. For example, pH 4 is ten times more acidic than pH 5 and 100 times (10 times 10) more acidic than pH 6. The same holds true for pH values above 7, each of which is ten times more alkaline (another way to say basic) than the next lower whole value. For example, pH 10 is ten times more alkaline than pH 9 and 100 times (10 times 10) more alkaline than pH 8.