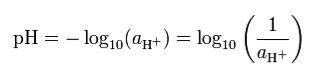Course:MATH110/Archive/2010-2011/003/Teams/Zug/Homework 13

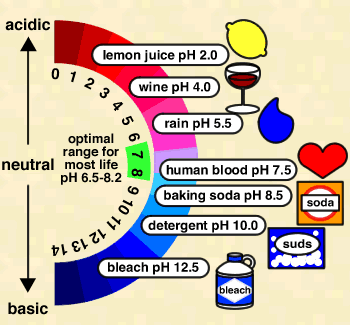
In measuring pH, we need to first understand what pH is exactly. pH is used in chemistry and is a measure of the acidity or basicity of an aqueous solution, furthermore it is an inverse logarithmic representation of Hydrogen proton concentration within solution (Covington et al 1985). From an applications standpoint, pH on a logarithmic scale will range from 0 to 14 where any value measured to be below 7 is considered acidic and any value above 7 is considered basic and 7 being neutral. Now it is important to understand that each increase or decrease in value does not mean that you are simply increasing or decreasing the pH by 1 value within the solution. Rather each individual pH unit is a factor of 10 relating to either it’s lower or higher neighbour. Consider pH of 3, if I was to increase the pH to 4, I would have increased the basicity of my solution by 10 (or ten times that of the previous pH value). Also, if I was to have a solution with pH 4 and I worked to decrease the pH to a value of 1 I would have seen a 1000 times drop in basicity (10^3). These observations can be fully supported by the equation:
The reasoning as to why pH scales are run on a logarithmic function can be traced to hydrogen ion concentration. Ion concentrations, more specifically hydrogen ions are incredibly small but addition or removal of these ions in just a small manner can work to significantly impact the acid or base. This significant impact can be traced to Hydrogen’s ability to donate electrons or receive electrons from other molecules within the solution and the subsequent electron flow will directly impact the pH of the solution by a large quantity given the fact that electrons carry a negative charge.
Below is the representation of values and their logarithmic counterparts:
Number Representation and Logarithms
References
Covington, A. K.; Bates, R. G.; Durst, R. A. (1985). "Definitions of pH scales, standard reference values, measurement of pH, and related terminology"