Course:FNH200/Lessons/Lesson 10/Page 10.4
10.4 Effects of Ionizing Energy Absorbed by Food - Preservation principle
The basis of food preservation by treatment with ionizing energy is the ability of the absorbed quanta of energy to dislodge electrons from molecules with the concomitant creation of free radicals without inducing radioactivity in the food.
When ionizing energy from a permitted source for food use is absorbed by food and collides with a molecule or atom, an ion-pair is produced if the energy from the collision is sufficient to dislodge an electron from an atomic orbit. This phenomenon can lead to breaking up of one or more bonds between atoms in the molecule, leading to new molecular fragments possessing unshared electrons (free radicals). Because of the unshared electron, free radicals are extremely reactive and tend to react with other free radicals or other molecules with unshared electrons.
It is believed that only one out of every six billion chemical bonds in bacteria or food molecules are broken by irradiation. However, the formation of ion pairs and free radicals, the reaction of free radicals with one another or other molecules, and the chemical and physical phenomena that occur as a consequence of these events form the mechanisms for the inactivation of microorganisms, enzymes and alterations of food constituents during food irradiation.
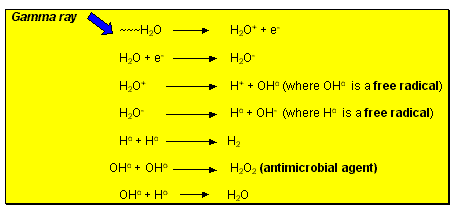
The changes induced in food by absorption of ionizing energy can arise from both direct and indirect effects. Please read the required reading Irradiation and Food Safety for details. You will note that many of the effects observed in foods arising from the absorption of ionizing energy are due to indirect effects as explained in the optional reading. This is shown below:
Hydrogen, hydrogen peroxide and hydroperoxy free radicals are produced when ionizing energy is absorbed by foods (fruits, vegetables, meats, fish) that contain substantial quantities of water. Figure 10.3 shows the reactions of hydrogen (H) and hydroxyl (OH) free radicals produced by gamma irradiation of water molecules. These free radicals only exist for about 0.0001 seconds, but generate hydrogen peroxide (H2O2) which is the antimicrobial agent that kills bacteria, yeasts, and moulds in foods. In many cases, the free radicals are formed within the microbial cells.
As mentioned earlier, microorganisms may also be killed by a "direct effect" of the ionizing energy upon genetic material within the microbial cells that leads to the death of the microorganism. As mentioned in the required reading (Irradiation and Food Safety) "the damage occurring from ionizing radiation can be random and extensive, making DNA repair near impossible". In some cases, even relatively small changes in the DNA can destroy bacterial cells, and the disruption of genetic material in living cells by irradiation also enables the destruction of insects, inactivation of parasites, delaying of ripening, and prevention of sprouting.
Are Free Radicals Unique to Irradiated Food?
A concern that has been expressed in regard to the use of food irradiation is the generation of free radicals during exposure of the food to ionizing energy.
It is true that free radicals are produced in foods during irradiation. However, the free radical formation is not unique to foods which have been irradiated with ionizing energy. For example, oxidative reactions in foods containing unsaturated fats also involve free radical formation, and free radicals are also formed during the course of the Maillard browning reactions. Free radicals are also produced within our bodies and other living tissues during normal metabolism. Mechanisms (chemical and enzymatic) for inactivation of free radicals exist within the human body and other living tissues.
Does irradiated food become radioactive?
Irradiation using approved sources provides enough energy to knock an electron from the outer orbit (that is why it is termed "ionizing radiation" or "irradiation"); however, it does not have sufficient energy to penetrate the nucleus and eject neutrons, which would be required to induce radioactivity. Therefore food will NOT become radioactive by irradiation conducted using approved energy sources and within the approved limit. To become radioactive, food would need to be exposed to a minimum of 15 MeV of energy. The energy output of Cobalt 60, Cesium 137, and e-beam accelerators is carefully regulated. The maximum energy outputs allowed are 5 or 10 MeV, which are too low to induce radioactivity in food.
You may be interested to know that all foods are naturally radioactive, although of course at a very low level. This low background level of radioactivity arises from the naturally occurring isotopes in elements such as carbon, phosphorus, potassium, and sulfur.