Course:FNH200/Lessons/Lesson 07/Page 07.3
7.3 Freezing and Frozen Storage of Foods
Freezing provides longer shelf life of food. It is carried out at temperatures well below 0°C. In fact, commercial freezing requires a minimum of -18°C. Household freezers only reach temperatures of -12 to -14°C
The basis for preservation by freezing/frozen storage is that freezing permits longer term storage than refrigerated storage due to:
- lower temperatures used (remember that microorganisms can not grow well at temperatures below -9.5°C)
- lower water activity (by freezing the "free" water present in the food)
Both factors slow down chemical and enzymatic reactions as well as microbial growth.
- Keep in mind that physical, chemical & enzymatic changes may still take place, especially if freezing and frozen storage conditions are not optimal.
- Freezing slows/stops microbial growth. However, storage of food at freezing temperatures does not kill all microorganisms and in fact many disease-causing and spoilage-causing microorganisms can survive in frozen foods for many years (e.g. Listeria monocytogenes). Once the food is thawed, the surviving microorganisms can resume their growth and function, causing disease or spoilage if the proper conditions for microbial growth prevail.
- When freezing of food is properly done, it can preserve the quality of the food without causing major changes in appearance, texture and flavour.
- Frozen foods are generally of higher nutritional and aesthetic quality than thermally processed foods. The faster the rate of freezing, the better the retention of quality, both from sensory and nutritional perspectives. The effect of freezing and other preservation methods on nutrient quality will be discussed later in the course (Lesson 11).
- Whether food is frozen in your home or in a food processing plant, the same principles govern the maintenance of quality during freezing and frozen storage.
What does "freezing point" mean?
Freezing point is the temperature at which ice crystals are in equilibrium with air-saturated water at 1 atmosphere pressure. Solutes in water will depress the freezing point.
What is the freezing point of pure water? ...of water in foods?
Although the freezing point of pure water is 0oC, actually water does not begin to freeze until it is supercooled to several degrees below 0oC.
The freezing point of foods is below the freezing point of pure water, because foods contain solutes dissolved in the aqueous (water) phase. The dissolved solutes have the net effect of lowering the freezing point of foods by several degrees Celsius.
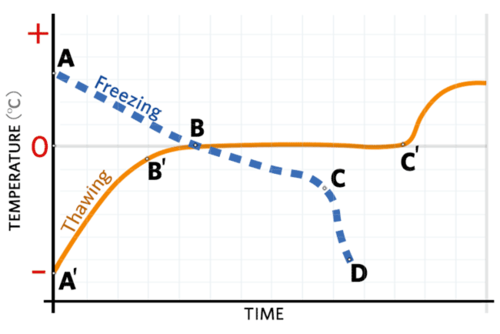
Once water starts to crystallize, there is an abrupt rise in temperature due to the evolution of the latent heat of fusion or crystallization. Only after all the water has frozen (crystallized) will the temperature approach the temperature of the freezing environment.
Note that water is frozen out as pure water. Until all of the water is frozen, there is an ever-increasing concentration of water-soluble solutes in the unfrozen phase. In fact, there is a considerable proportion of unfrozen water at temperatures below 0 C.
How much water remains unfrozen?
This depends both on the temperature as well as the food product itself. For example, for beef, 70% of the water remains in the unfrozen state at -4.0 oC, compared to 3% and only trace amounts at -9.0 and -18oC, respectively.
From this description you will begin to appreciate that many complex changes occur within food systems as the freezing operation is carried out.
Freezing and thawing curves are shown below in Figure 7.1. This figure describes the transitions that take place in a hypothetical food material during freezing and thawing, including:
- removal of heat from the product (sensible heat)
- freezing of water (liquid) into ice crystals (solid) (latent heat of fusion or crystallization)
- further cooling to the surrounding temperature
Changes in Food During Freezing, Frozen Storage and Thawing
You may have had the unfortunate experience of placing a beverage or other container of liquid food in a freezer to chill it quickly, only to find the container has cracked or the lid popped off because of the expansion of the water in the food as the water froze. The same phenomenon occurs within the cells of food during freezing if the conditions are not carefully controlled.
Rupture of cell walls and membranes during freezing and thawing can lead to formation of drip when the product is thawed. The process of freezing foods has great influence on the ultimate quality of the food once it is thawed and prepared for consumption. Changes that occur in foods during freezing, storage and thawing can be both chemical and physical in nature.
Various chemical, enzymatic and physical changes are promoted as a result of the concentration of components (concentration effects) in the unfrozen water phase within the frozen foods.
For example:
- Chemical changes such as oxidative rancidity or oxidation of flavour components, pigments and vitamins.
- Enzymatic reactions such as enzymatic browning or lipolytic rancidity.
- Meats become tougher due to protein denaturation by chemical effects and cell breakage by ice crystals
In freezing foods, the objective is to promote the formation of tiny ice crystals rather than the formation of fewer but larger ice crystals that cause cellular damage. Ice crystal damage can lead to loss of water from the food product once it is thawed.
- The drip that is found in thawed strawberries or beef is due in part to ice crystal damage to the cells, leading to leakage of cellular fluids into extracellular spaces, and to the loss of water-holding capacity of food components as a result of concentration effects.
- Emulsions and other dispersions are destabilized by the growth of numerous small ice crystals to larger, less numerous but more damaging ice crystals; such growth in ice crystal size is usually caused by temperature fluctuations.
- You may have noted shrinkage and development of graininess in ice cream stored in the frost-free freezer section of your refrigerator. This is due to partial melting of the ice cream during temperature fluctuations that result from the defrost cycles. This leads to foam destabilization and crystallization of lactose as a consequence of concentration effects.
Other undesirable changes include formation of package ice and freeze dehydration which is popularly called freezer burn and can produce unsightly food surfaces and loss of nutrients. "Freezer burn" is a misnomer since the food does not "burn" in the freezer but rather takes on an appearance of having been burnt because of the moisture loss that occurs during this freeze dehydration.
How can we minimize Changes in Food During Freezing, Frozen Storage and Thawing?
- Blanching (lesson 6)
- Proper temperature control for freezing and frozen storage
- Appropriate packaging (discussed later in this lesson)
Factors affecting the quality of frozen foods
The type and extent of changes during freezing, frozen storage and thawing, which are directly related to the final quality of frozen foods, are affected by many factors. Here are the 4 most common ones:
- rate of freezing
- final storage temperature
- stability of storage temperature, and
- rate of thawing
Rate of freezing (freezing rate)
The rate of freezing of foods is very important, with rapid freezing rates being desirable since the formation of many small ice crystals is favoured. Freezing rates vary depending on:
- Food composition: Some food components such as proteins and fats act as insulators. Presence of these components slow down the freezing.
- Temperature difference: the greater the temperature difference between the food and the refrigerant, the faster the freezing rate.
- Product thickness/geometry and heat transfer rate: the thinner the food piece or greater the heat transfer rate, the faster the freezing rate.
- Air velocity: the greater the velocity of refrigerated air or circulating refrigerant, the faster the freezing rate.
- Degree of contact: the more contact between the food and the cooling medium, the faster the freezing rate.
These five factors are given great attention in the design of food freezing plants in order to maximize the rate of freezing so that quality attributes of the food commodity can be retained.
Final storage temperature
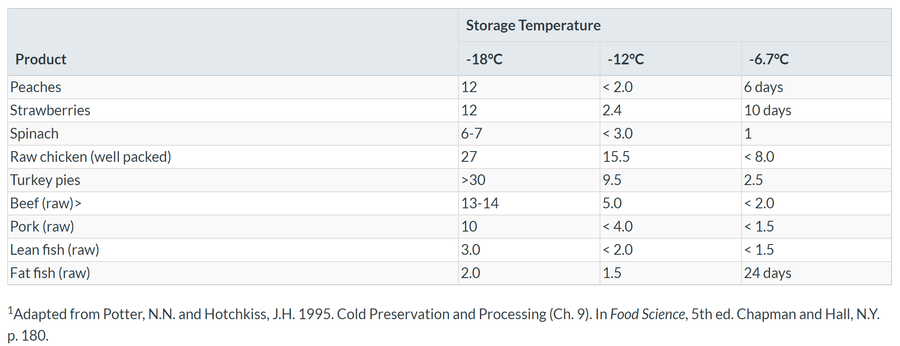
The final temperature for storage of frozen foods is dictated by a number of factors: texture changes, chemical reactions, etc. There are a number of reasons why the storage temperature of about -18°C is commonly used and the normal operating temperature of deep freezers sold for home use is also -18°C.
Table 7.1 shows the number of months during which high quality storage life of frozen food can be maintained at three different storage temperatures. Note how temperature has a marked influence on the storage life of frozen foods. You can see in Table 7.1 that each food commodity has its optimum storage life at -18°C. The rates of deterioration of frozen foods are governed by the chemical composition and physical structure of the foods.
Stability of the storage temperature
As important as the storage temperature is the stability of the storage temperature. It is important to note that as the temperature of the frozen food increases, the amount of unfrozen water increases. Thus small fluctuations in storage temperature can cause melting of small ice crystals with subsequent refreezing of the liquid water on to other small ice crystals as the temperature drops, leading to the formation of fewer but larger ice crystals which can produce negative changes in the food quality.
| Want to learn more? |
|
The rate of thawing
The rate of thawing of frozen foods is as critical to quality maintenance as the rate of freezing. Maximum quality retention is achieved by rapid thawing rates. Figure 7.1 shows a thawing curve for a hypothetical food product. Since ice is a good conductor of heat (it has a high thermal diffusivity) the temperature of a frozen food rapidly approaches the melting point of ice. After the rapid initial temperature increase, subsequent increases in temperature occur very slowly because of the need to supply the latent heat of fusion1 for the conversion of water from the crystalline state to the liquid state at 0°C.
1Latent heat is the quantity of heat required to change the state or condition under which a substance exists, without changing its temperature.
| Want to learn more? |
|
Freezing Methods
The three basic methods of freezing foods are presented below.
Table 7.2. Commercial freezing methods.
| Air Freezing | Indirect Contact Freezing | Immersion & Cryogenic Freezing |
|---|---|---|
| Still-air "sharp" freezer
Air blast freezer Fluidized-bed freezer (IQF) |
Single plate
Double plate Pressure plate Slush freezer |
Heat Exchange fluid
Compressed gas Refrigerant spray |
| Adapted from: Potter, N.N. and Hotchkiss, J.H. 1995. Cold Preservation and Processing (Ch. 9). In Food Science, 5th ed. Chapman and Hall, New York, NY. p.187 | ||
Air Freezing
Air freezing is the oldest and most common type of freezing used. The freezer section of your household refrigerator and the deep freezer are examples of still air freezers or low air velocity systems
Air blast freezing
Air blast freezing is a moderately fast freeze because of vigorous circulation of cold air. The product is placed on trays or mesh belts and passed slowly through an insulated tunnel. In different systems the temperature may range from -18°C to -34°C, with an air velocity of 100-3500 lineal feet per minute, with a counter current air flow.
Air blast freezers operate at lower temperatures than still air freezers and rely on movement of the cold air at high velocity over the food in order to achieve rapid removal of heat and to maximize the freezing rate.
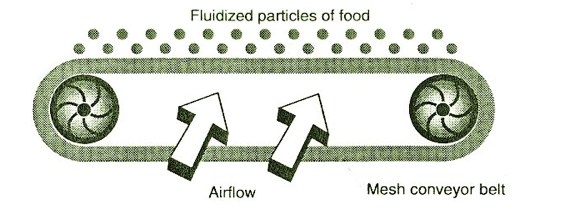
Garden peas and individually quick frozen shrimp or prawns are frozen in fluidized-bed freezers. In this type of air freezing, solid particles ranging in size from peas to strawberries are being exposed through a movement of the cold air (-20 to -34°C) at high velocity as they pass along a conveyor belt. This will impart a vibratory motionto food particles, accelerating the freezing rate. The cold air being forced upward through the bed lifts and suspends the food particles, thus fluidization occurs. In this way, a rapid freezing rate is accomplished and an IQF (individually quick frozen) product is produced. In other words, food items are frozen as individual pieces and are not stuck together.
The garden peas, corn and other IQF products are packaged after freezing. If garden peas, or any other food product that is stated to be IQF on the package label, are present in the package as a solid block, this indicates that the product has undergone partial thawing and refreezing during storage, distribution, retailing, or on the way home from the retail store to your home.
Figure 7.2 illustrates the process of "fluidized bed freezing" used for some products.
Video
Corn Processing
Please watch the video on Corn processing. Note the following:
- How are the kernels separated from the cobs?
- Why is corn starch added in the "froth flotation washer"?
- What is the purpose of blanching? what temperature/time is used?
- What is the "IQF" process and what temperature is used for freezing?
- How long can corn be stored in a frozen state?
Indirect contact freezers
Indirect Contact freezers are used in the production of various frozen food commodities. In these freezers, food is placed on belts or trays and a refrigerant circulates through a wall beside the food. As the food comes into "contact" with the cold wall, it quickly cools down and freezes. Plate and slush freezers are some examples of indirect contact freezers.
During plate freezing food products are placed in contact with a metal surface which is cooled by a cold brine, or a vaporizer refrigerant such as ammonia. The packaged food either rests on, slides against or is pressed between the cold metal plates. These plates maintain firm contact with two major surfaces of packages to facilitate heat transfer and prevent bulging of the packages during the freezing process. Fish sticks and frozen fish fillets are commonly frozen in plate contact freezers (Figure 7.3).
Another type of indirect contact freezer is the slush freezers or scraped surface heat exchangers. These freezers can be used only for fluid food products. A common example of a scraped surface freezer is the machine used to convert ice cream mix to soft ice cream in restaurants and ice cream shops. The same principle is used in the commercial production of ice cream that is sold as hard ice cream. In the case of ice cream, the rotator not only aids in promoting rapid freezing and the development of small ice crystals, but it also aids in the incorporation of air bubbles into the freezing mix which results in the formation of a solid foam (review definition of a solid foam from Lesson 2).
As mentioned before, shrinkage and development of graininess in ice cream stored in the frost-free freezer section of your refrigerator is due to partial melting of the ice cream during temperature fluctuations that result from the defrost cycles. This leads to foam destabilization and crystallization of lactose as a consequence of concentration effects.
Video
Ice Cream Processing Video
Please watch the video on Ice cream production. Note the following:
- Main steps followed in the production of ice cream
- What type of freezer(s) are used?
- What tests are conducted to assure safety and quality?
Immersion and Cryogenic freezing
Immersion freezing involves the immersion of packaged or un-packaged food products directly in a non-toxic refrigerant fluid. The refrigerant fluids commonly used are propylene glycol, glycerol, sodium chloride, calcium chloride, and mixtures of salt and sugar. Canned citrus juice, turkeys and chickens are often frozen in immersion freezing units. Ice cream popsicles can also be frozen using this method.
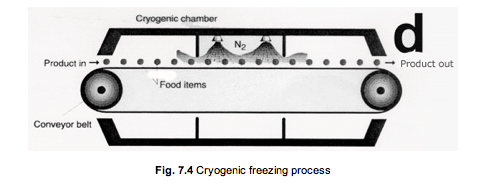
Cryogenic freezing is accomplished with cryogenic liquids, with liquid nitrogen being the most commonly used. This is a very rapid freezing method in which un-packaged or thinly packaged foods are exposed to extremely cold freezant. In contrast to the liquid immersion freezing, heat removal is accomplished during a change of state by the freezant. Products such as TV dinners, preformed hamburger patties and other high value food products are frozen in cryogenic freezers because of the excellent retention of quality imparted by the rapid rate of freezing and small ice crystal formation. Figure 7.4 illustrates a cryogenic process.
How does liquid nitrogen cryogenic freezing work?
The product is first placed on a conveyor belt and is moved into the pre-cooling part of the freezing unit. Once the food is cooled, the food is sprayed by liquid nitrogen as it is being moved through the conveyor belt; here is where the freezing process takes place, by the nitrogen boiling as it contacts the food. Finally, the food is allowed to equilibrate to the desired final temperature (between -18°C to -30°C).
The following table gives a summary of some of the advantages and disadvantages of the freezing methods we have discussed:
Table 7.3. Some advantages and disadvantages of freezing methods.
| Freezing method | Advantages | Disadvantages |
|---|---|---|
| Air blast freezing; Fluidize-bed freezing (IQF) |
|
|
| Indirect Contact Freezing |
|
|
| Immersion/ Cryogenic freezing |
|
|
| Want to learn more? |
|
Packaging Considerations for Frozen Foods
Packaging materials to be used for frozen foods must be resistant to the transfer of water vapour from the food to the dry environment within the freezing unit. The packaging material must not shatter in the cold temperatures encountered in frozen storage. Therefore glass is not a good material for packaging frozen foods because it tends to shatter and it is not flexible. The packaging material should resist the formation of pinholes during normal handling.
Breaches in the packaging material will promote the development of freezer burn on the exposed areas of the food. Depending on the food, the packaging material may have to possess barrier properties toward light and/or oxygen.
| Want to learn more? |
|