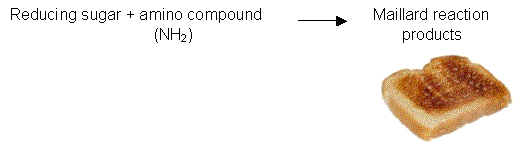Course:FNH200/Lessons/Lesson 02/Page 02.2
2.2 Food Component
Foods are made of chemical components that are working together and making the food the way it is. Chemical composition is determination of these compounds. The chemical composition tables identifies the amounts of these compounds in each food.
In Canada, the Canadian Nutrient File, provides a data base of foods and their listed composition. https://food-nutrition.canada.ca/cnf-fce/index-eng.jsp
Quantifying the amount of Carbohydrate, Fat, Protein, Water and Ash is called proximate analysis.
Food components can be classified as major and minor components.The major food components of food systems are carbohydrates, fats, proteins and water. There are also minor food components, organic acids, pigments, aroma compounds, vitamins and minerals. In this section we will discuss the important functional properties of these components and how those properties influence the chemical and physical properties of foods.
2.2.1 Food Major Components
The major food components of food systems are carbohydrates, fats, proteins and water. These are the compounds are found in largest amounts in foods. Each component has variety of functional properties which affects the physical and sensory characteristics of the food during processing and storage. Understanding these properties is essential tool in product development and also quality control. Please note that, although these components may be similar to what nutritionists and dietitians call macronutrients, we are looking at them from food science perspective.
2.2.1.1 Carbohydrates
Terms to remember |
|
Carbohydrates are one of the three main classes of nutrients (the other two being fats and proteins). They occur in foods as sugars and starches and are the human body's main source of energy. Digestible carbohydrates contribute 4 Calories (kilocalories) of metabolized energy per gram. Carbohydrates should contribute about 50% of our caloric intake per day; and most of the carbohydrates that we consume should be in the form of complex carbohydrates (polysaccharides) such as starch rather than as simple carbohydrates (monosaccharides and disaccharides) such as table sugar.
a. Monosaccharides
The main monosaccharides found in foods are glucose, fructose and galactose. These are referred to as simple carbohydrates and one of their main functions is their ability to impart a sweetness sensation; however, sugars vary in their sweetening power. The sweetness of various sugars in comparison to sucrose (table sugar) is shown in Table 2.2
b. Disaccharides
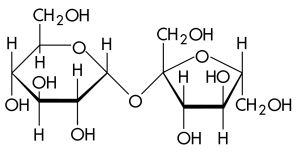
Disaccharides are formed by the union of two monosaccharide molecules. Disaccharides are also considered as "simple" carbohydrates. They can be split into their component monosaccharides by enzymes or by boiling with dilute acids. The most important disaccharides in foods are sucrose, lactose and maltose. These disaccharides differ from one another in solubility, sweetness, and other properties.
Sucrose
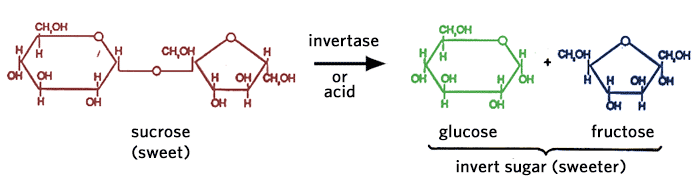
Table sugar, obtained from sugar-cane or sugar-beet, is mainly pure sucrose. It is formed from glucose and fructose linked together. Sucrose can be found in a variety of fruits, grasses and roots.
One of the recent trends in the food industry, particularly for carbonated beverages, is the use of invert sugar in place of sucrose because of the inherently greater sweetening power per unit weight of the fructose containing sweetening systems (see Table 2.2). Invert sugar is produced by hydrolyzing sucrose with the enzyme invertase or with acid, to produce a mixture of glucose + fructose (1:1).
Incidentally, the primary sugars in honey are glucose and fructose in a 40:60 ratio. Most of the nectar collected by the honey bee contains sucrose which is hydrolyzed by invertase in the saliva of the honey bee. Some of the glucose is converted to gluconic acid and hydrogen peroxide by glucose oxidase, another enzyme secreted into the collected nectar by the honey bee. The gluconic acid and hydrogen peroxide act as preservatives in the nectar. Honey also contains minute quantities of disaccharides and complex sugars.
Table 2.2. Relative sweetness of carbohydrate sweeteners.
| Sugar | Sweetness Index* |
|---|---|
| sucrose | 100 |
| glucose (dextrose) | 70 - 80 |
| fructose (levulose) | 140 |
| invert sugar | 100 - 130 |
| corn syrup (mixture of glucose, maltose | 50 |
| maltose | 20 |
| lactose | 10 - 20 |
| galactose | 60 |
| sorbitol | 50 |
| xylitol | 100 |
| high fructose corn syrups: | |
| 42% fructose | 100 |
| 55% fructose | 100+ |
| 90% fructose | 120 - 160 |
| *Perceived sweetness of a sweetener compared to sucrose as a reference.
Adapted from: Desrosier, N. W. 1976. Elements of Food Technology. AVI Publishing Company. Westport, CT. Pomeranz, Y. 1985. Functional Properties of Food Components. Academic Press Inc., Orlando, Fl. | |
It is important to note that sweetness has no relation to caloric contribution of a sweetening agent to the diet. Fructose and lactose each produce 4 Calories of metabolized energy per gram when digested and absorbed, but lactose is only one-seventh as sweet as fructose. Thus for an equivalent sweetness intensity, less fructose would be required than lactose. Conversely, a product sweetened with lactose could potentially contain seven times the caloric content compared to a product sweetened with fructose.
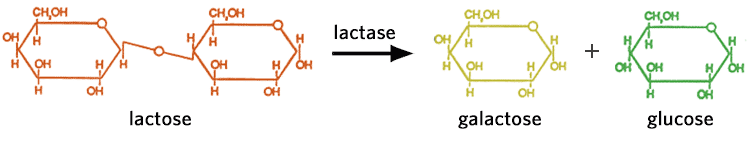
Lactose
Lactose, also known as milk sugar, occurs in the milk of all animals. Cow's milk contains about 4-5%, whereas human milk contains 6-8% lactose. Lactose is formed by linking glucose and galactose together. The hydrolysis of lactose found in dairy products into its component monosaccharides is catalyzed by the enzyme lactase. Breaking down lactose substantially increases the sweetness. Lactose can also be fermented by lactic acid-producing bacteria, into lactic acid. This is the acidulant and preservative agent in yogurt and numerous cheeses.
Have you seen lactose-free products in the market or have you met someone who is "lactose intolerant"?
Lactose intolerant people are those who do not have the enzyme lactase necessary to digest (breakdown) lactose (milk sugar). People who are lactose intolerant can suffer from minor cramps to extreme intestinal discomfort. Lactose-free products have had the enzyme lactase (usually isolated from yeast) added to them. Alternatively, lactose intolerant individuals can take tablets containing the enzyme, prior to eating or drinking dairy or other food products with lactose or milk solids.
Maltose
The sugar maltose contains two glucose units linked together. It is obtained when starch (eg corn starch) is hydrolysed by the enzyme amylase or by heating with dilute acid (Figure 2.4). Maltose can be further hydrolysed by the enzyme maltase into its component D-glucose units, which are then enzymatically isomerized by the enzyme glucose isomerase to produce a liquid syrup composed of 42% fructose, commercially known as high fructose corn syrup (HFCS 42). HFCS has 42% fructose, 52% glucose and 6% starch. Subsequent technological improvements in which the syrup is passed through an ion-exchange column that retains fructose, allow for the production of a 90% fructose syrup. Today, HFCS 90 is blended with HFCS 42 to create HFCS 55, which has a sweetness profile similar to sucrose (Table 2.2). Many soft drinks are now sweetened with HFCS especially when cost of these syrups is lower than the cost of sucrose or even invert sugar.
Functional Properties of Simple Sugars in Foods
The functional properties of sugars in foods are summarized below:
- Sugars are widely used for their sweetening power. The sweetness of carbohydrates is determined by their molecular structure and interaction with sensory receptors on the tongue. Simple sugars vary in their sweetness (Table 2.2)
- Sugars produce body and mouth feel when they are incorporated into foods at concentrations high enough to affect the viscosity (resistance to flow) of the food product
- production of hot supersaturated sugar solutions with controlled crystallization during cooling is the basis of formation of many hard candy products, toffees and related products.
- sugars are readily soluble in water because they contain many hydroxyl (OH) groups, which form hydrogen bonds with water. Solubility of sugars increases as the temperature of water increases. This property is used to produce syrups of varying concentrations for various uses (e.g. pancake syrup, concentrated syrups for use in food processing, cooking or confections.)
- Sugars can be crystallized from solution when water is evaporated. This is the basis of production of table sugar (sucrose) from the juice extracted from sugar cane and sugar beets.
- Sugars, in sufficiently high concentration, can be used to inhibit growth of undesirable microorganisms. They function as a preservative by binding water needed by the microorganisms.
- sugars are fermented by microorganisms with the concomitant production of acids and/or alcohol as well as flavouring compounds. This is the basis for production of fermented foods and ingredients obtained by means of microbial fermentations.
- sugars caramelize when exposed to high temperatures. See "Browning reactions" below.
- reducing sugars react with proteins and amino compounds to produce flavours and colours in foods (Maillard browning). See "Browning reactions" below.
Browning reactions
Another important property of the simple sugars is their ability to serve as reactants in non-enzymatic browning reactions, namely caramelization and the Maillard browning reaction.
The caramelization reaction involves reaction of sugars (reducing and non-reducing sugars) when heated at high temperatures (200°C) to produce caramel and butterscotch flavours. The brown pigments formed during the heating of sugars contributes to the colour of caramel candies and toffees (Figure 2.5a). The pigments are not the same as the melanoidins formed during the Maillard reaction.
The Maillard browning reaction occurs when reducing sugars react with nitrogenous compounds such as amino acids, proteins or amines (Figure 2.5b).
- A reducing sugar contains a free aldehyde or ketone group. Therefore, it will contain a “free” OH on the position next to the O in the ring structure
The Maillard browning reaction is responsible for the formation of the brown pigments that appear on bread slices when they are toasted in the toaster.
| Want to learn more? |
|
The Maillard browning reaction is responsible for the formation of the brown pigments that appear on bread slices when they are toasted in the toaster.
Figure 2.5c. Maillard browning reaction. Toasted bread is an example of desirable flavours and colours produced from this reaction.
| Want to learn more? |
|
Many low molecular weight intermediate compounds are formed and these often are aroma and flavour compounds that contribute to the desirable or undesirable flavours produced in a food by the Maillard reaction. Examples of desirable compounds are the aroma and flavour of baked bread, toasted bread and roasted coffee, while undesirable aromas and flavours are those that form in skim milk powder during storage or during the browning of canned peaches during long-term storage. The brown colours are high molecular weight pigments, melanoidins, formed as a result of polymerization of some of the low molecular weight intermediate fractions.
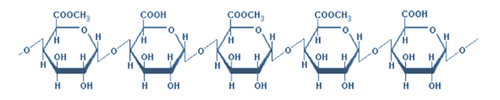
c. Polysaccharides
Terms to remember |
|
Polysaccharides are high molecular weight, long chains of monosaccharide units (i.e. glucose). They are classified as the complex carbohydrates and differ from simple carbohydrates by being insoluble in water and generally tasteless. Most of the polysaccharides used in food products are derived from plant or seaweed sources; a few are from microbial origin. They contribute to the thickness or viscosity and textural properties of food products.
| Polysaccharide | Characteristics and Functional Properties |
|---|---|
| pectins |
|
| agar |
|
| alginates |
|
| gum arabic or gum acacia |
|
| carrageenan |
|
| xanthan gum |
|
| cellulose and hemicellulose |
|
| starch |
|
Starch
Starch is made out of polymers of glucose joined by an alpha, 1-4 link. A single molecule of starch can include anywhere from 400 to several hundred thousand glucose units. Therefore, although they are made of long chains of sugar molecules, starches do not elicit a sweet taste and rather taste quite "bland". The length and bulkiness of the starch molecule prevent it from interacting with our tongue receptors! In food, starch is mostly used as a thickening, suspending and gelling agent.
In foods such as cereals and tubers starch exists in the form of starch granules (Figure 2.6). Starch molecules (amylose, a straight/linear chain starch molecule and amylopectin, a branched starch molecule) are tightly packed within starch granules. The starch granule is not digestible, nor is it soluble in cold water unless it is heated.
Figure 2.7
When starch is heated in water, it undergoes a phenomenon known as gelatinization. The starch granules absorb water and swell-up as the water entering the granule begins to "loosen" the bonds between the starch molecules. Hydrogen bonds form between the water and starch molecules. The starch granule eventually "bursts", becoming soft and pliable. This is the phenomenon that occurs when puddings are made or when flour is used as a thickening agent when making gravies. Starch gelatinization is the phenomenon that leads to the conversion of hard, unchewable, raw rice kernels to the soft, easily chewed, cooked rice.
Gelatinized starch can lose some of its water holding capacity upon cooling and/or during refrigerated storage. This phenomenon is known as retrogradation, and involves the re-association of starch molecules, especially the amylose polymers, into an ordered structure. The linear amylose molecules orient themselves in crystalline regions, leading to a squeezing out ("syneresis") of water and a loss of tenderness of the food (e.g. staling of bread) or the development of a gritty texture (e.g. starch based pudding stored in the refrigerator).
It is interesting to observe that bread stales more quickly in the refrigerator than the freezer or at room temperature. Can you think of why this is the case?
Retrogradation can be avoided to a certain extent through the use of dextrins and/or modified starches, thus reducing the tendency for alignment of linear amylose chains. Starches can be partially hydrolysed by acids or enzymes to produce products of intermediate chain length (dextrins) that have numerous uses in food products. Some of the dextrin products are used to create foods that provide the sensation of containing fat but that are low in fat.
Retrogradation can also be partially reversed by heating the food (e.g. heating stale bread or buns in an oven or the microwave oven); however, once the product cools the starch quickly retrogrades again.
Cellulose and hemicellulose
These polysaccharides are present in many plant tissues as supporting structures (e.g. the fibres in celery). They too are polymers of glucose but joined by a beta, (ß)1- 4 link. However, humans do not have the enzyme needed to break the beta link and therefore, cellulose is indigestible. Along with pectin and the other carbohydrate gums, cellulose forms the indigestible portion of our carbohydrate intake that is known as dietary fibre.
Xanthan Gum
Xanthan gum is a polysaccharide with a ß-D-glucose backbone like cellulose, but every second glucose unit is attached to a trisaccharide.
Xanthan Gum is produced by the bacterium Xanthomonas campestris, which is found on cruciferous vegetables such as cabbage and cauliflower and causes black rot. Nowadays, it is cultured in large fermentation tanks and purified. Xanthan gum is used in salad dressings as a thickening agent, which enables the dressing to cling to the salad components. It is also used as a thickener for sauces, to prevent ice crystal formation in ice cream, and as a low-calorie substitute for fat.
Pectin
Pectin is a polysaccharide that acts as a cementing material in the cell walls of all plant tissues. The white portion of the rind of lemons and oranges contains approximately 30% pectin.
It is used in jams and jellies as a gelling agent. It also contributes to the viscosity of tomato paste and ketchup. Pectins will give contribute to the mouth-feel of foods, and help maintain particles in suspension (e.g. orange juice, unclarified apple juice).
Agar, Alginates & Carrageenan
These polysaccharides are extracted from different types of seaweed (kelp). In general, they are used as thickening, gelling and suspending agents.
For example, alginates keep solids and liquids in suspension in fruit juices and provide thickness to dietetic and regular salad dressings, puddings, pie fillings, ice cream, sherbet and icings. Carrageenan is used as a suspending agent to keep cocoa particles in suspension in chocolate milk, and it is also used as a stabilizer in ice cream (stabilizing the colloidal dispersions).
Gum Arabic (Acacia Gum)
These gums are plant exudates from the bark of the acacia trees. It is used as thickener and stabilizer in products like beer, soft drinks, and ice cream.
| Want to learn more? |
|
2.2.1.2 Fats
Terms to remember |
|
You will recall from lesson 1, that consumers are demanding "healthier" foods. Many of the products we currently see in the market, have selling points such as "low-fat" or "fat-free". This general trend towards low-fat products has given fats a negative reputation. Fats and oils are part of a group called lipids. Lipids can be found in the form of triglycerides, phospholipids and sterols. However, triglycerides make the largest class of lipids as most of the fats and oils we consume from food are in the form of triglycerides.
| Want to learn more? |
|
Functional Properties of Fats in Foods
The required reading talked about fats and the important functional properties they have by influencing the flavour and texture of foods. Fats are even necessary for the absorption of fat soluble vitamins. The functional properties of fats (and oils) in foods are summarized below:
- Fats act as a lubricant in food making the food more palatable and easier to chew and swallow.
- Fats have tenderizing power because they coat the flour particles (protein and starch) in baked goods, creating a flaky, lighter texture that makes them easy to tear apart. Fats work best as shortening when the crystals are in the beta prime form, which produces a fine texture in the baked goods. Cakes will have a crumbly texture without the moistness given by fats.
- Another function of fats in baked goods is the one called "aeration". Fats add air (gas) to batter and doughs. The fat surrounds the air molecules that are being incorporated into the batter. They contribute to the formation of the dispersion by decreasing the viscosity in the batter, thus making it easier to flow and rise.
- Fats and oils are carriers of many aroma constituents in foods that are usually fat-soluble. Thus, fats contribute to the overall flavour of food (we will review aroma and flavour in Lesson 3- Sensory perception of foods)
- Fats and oils can be heated to very high temperatures before they begin to smoke and vaporize. Foods fried in hot fats and oils (deep fat frying) cook very fast because of the temperatures that can be attained.
- Fats gradually soften when heated. This contributes to the desirable features such as chocolates that melt in your mouth and butter and margarines that are spreadable.
- Fats form part of emulsions (review colloidal dispersions) by acting as the dispersed phase or continuous phase. Some fats can also act as "emulsifiers", assisting in keeping the emulsion stable (see "Fats and their role in emulsions" below).
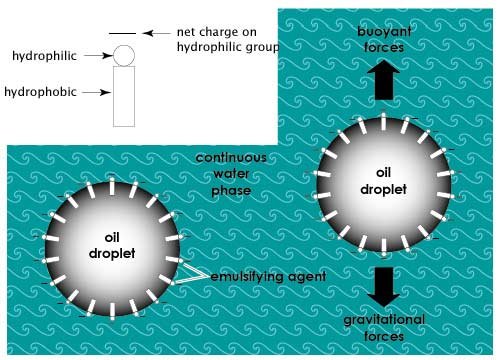
Fats and their role in emulsions
You will recall that there are two types of emulsions (see colloidal dispersions and Figure 2.2): water in oil [e.g. butter, margarine], and oil in water [e.g. mayonnaise, homogenized milk].
Homogenization or other high-energy mixing processes may be used to disperse one liquid phase into another. Nevertheless, after two liquid phases such as oil and water are mixed and then left to stand, the natural tendency is for the two phases to separate. Emulsifiers are compounds that promote the formation of emulsions, i.e., the dispersion of one phase in the form of small droplets, in the second continuous phase.
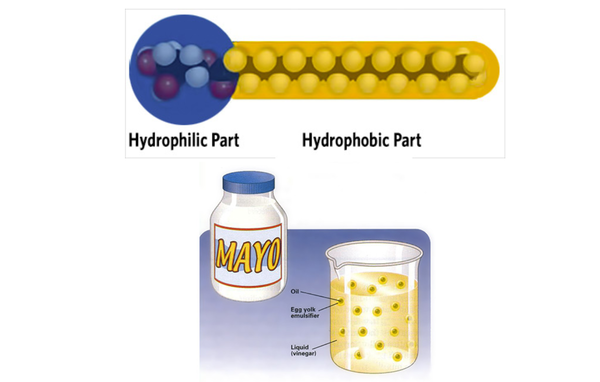
Certain type of fat molecules called phospholipids, can function as emulsifiers. Phospholipids are structurally similar to triglycerides, except that only two fatty acids are linked to the glycerol (making it a diglyceride), and a charged group (negatively charged phosphoric acid esterified with positively charged choline group) is linked to the third position of glycerol.
Lecithin is an example of a phospholipid. Lecithin is a naturally occurring emulsifier commonly found in egg yolk and soybean oil. Other naturally occurring emulsifiers include the proteins from milk, egg yolk or other foods. Sometimes, synthetic emulsifiers (e.g. "Polysorbate 60") are used to assist in forming food emulsions.
Emulsifiers are amphiphillic molecules that have a hydrophilic [water loving] portion and a hydrophobic [water hating] portion. Emulsifiers assist in formation of an emulsion by orienting themselves at the interface between the two phases, with their hydrophilic and hydrophobic portions facing water and oil, respectively, thereby reducing the interfacial tension between oil and water phases. This can also help to stabilize the emulsion by preventing the dispersed oil droplets or water droplets from coalescing together. Other factors that affect emulsion stability are droplet size, and the viscosity of the continuous phase. Droplet size must be such that, the downward pull of gravity, is balanced by the upward forces of buoyancy. This will reduce the tendency for "creaming" (floating to the top) of the less dense (oil) phase.
Note the difference between "Emulsifiers" and "Stabilizers". Stabilizers are compounds that increase the viscosity of the continuous phase, keeping the droplets suspended or dispersed and thus reducing the rate of creaming. Some of the polysaccharides discussed earlier in this lesson are commonly used as stabilizers to thicken the continuous phase (water); some examples are “xanthan gum” and “propylene glycol alginate”.
Figure 2.6 illustrates the function of an emulsifier in an oil-in-water emulsion.
Whant to learn more? |
|
The major function of milk homogenization is the formation of a stable emulsion to prevent fat separation, such as that which occurs in non homogenized milk.
Whant to learn more? |
|
2.2.1.3 Proteins
Terms to remember |
|
Protein molecules are made up of long chains of hundreds or even thousands of amino acid units joined together. Amino acids are a type of organic acid. They are made up of an amino group (NH2) and a carboxyl group (COOH) attached to the same carbon atom.
There are 20 different amino acids in proteins found in food systems and in the human body. Nine of the amino acids cannot be synthesized by human tissues and must be obtained via food. These essential amino acids are isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine; histidine (essential for infants only).
Adults require 0.8 grams of protein per kilogram of body weight. Protein consumed in excess of body requirements is converted to energy or is converted to fat for storage. Proteins produce 4 Calories per gram when they are digested and the amino acids are metabolized for energy.
Functional Properties of Proteins in Foods
Proteins in tissue systems such as meats and fish contribute to the texture of the products. The difference between a tender steak and a tough steak can often be related to the types and relative abundance of various types of protein molecules within the muscle structure. Proteins from various sources (cereal grains, milk, meat, fish, legumes) can be used in various states of purity as food ingredients with differing functional properties. Some of those functional properties are described below.
Emulsion formation
Many proteins are amphiphillic molecules as they contain hydrophilic and hydrophobic portions (from amino acids) allowing them to act as emulsifiers. One part of these amino acids is attracted to water, forming hydrogen bonds, while the other part avoids water and binds with oil.
- Egg yolk and mustard proteins in mayonnaise function as emulsifiers.
Foaming
- Proteins have the ability to trap air in bubbles and this leads to the formation of foams.
- Egg white proteins function as foaming agents in the making of whipped egg whites. Whipping introduces air and denatures (unfolds) the protein molecules. The protein molecules then coagulate to form a fine film around the air pockets.
- Solid foams such as meringue are formed when the whipped egg whites are heated causing the protein to denature and form a more rigid three-dimensional structure, which won't collapse when the air escapes.
- Bread and ice cream are also examples of solid foams. Ice cream is also a solid emulsion.
 Gluten, a protein in wheat flour, traps air bubbles in bread making. Bread was prepared and photo was taken by Morgan Reid, LFS, UBC. This bread was prepared using a 250-year old sourdough culture. More details on bread culture will be discussed in Lesson 09.
Gluten, a protein in wheat flour, traps air bubbles in bread making. Bread was prepared and photo was taken by Morgan Reid, LFS, UBC. This bread was prepared using a 250-year old sourdough culture. More details on bread culture will be discussed in Lesson 09.
Gel formation
- Gelatin (from the animal protein: collagen) forms a gel by trapping large volumes of water within a semi rigid three dimensional protein matrix.
- Heating of meat proteins during the manufacture of luncheon meats, such as bologna and frankfurters, leads to gelation and formation of the textures characteristic of cured meats.
- Products such as frankfurters and bologna are also emulsions.
- Milk protein also forms a gel when it is acidified, such as in making of yogurt and cheese. The gel holds water and has a smooth texture.
The Amazing Functionalities of Milk Proteins
- In latte (A): the milk protein traps air bubbles to form the foam structure.
- In milk (B): casein, a milk protein, acts as an emulsifier preventing the fat globules to separate (cream) from the skim milk portion. ( This function can be further enhanced by homogenization-will be further discussed in Lesson 6)
- In cheese (C): casein forms a gel structure in the cheese curd.
Proteins that function as enzymes
- Enzymes are proteins that function as biological catalysts.
- In some cases enzymes are added to food as an ingredient (invertase in candy making)
- Some enzymes are used to promote food-processing operations while others are causative agents in food spoilage (amylase can ruin a starch gel; lipases can cause lipolytic rancidity which is the release of free fatty acid from glycerides).
- Enzymes in living tissue food systems such as fruits and vegetables are responsible for the reactions associated with ripening. Those same enzymes will continue the ripening process after harvest and unless they are inactivated the enzymes will eventually cause spoilage of the product (e.g. loss of crispness of stored apples; loss of sweetness of apples during storage; loss of colour in the skin of apples during storage).
- Many heating processes in food processing are designed to inactivate enzymes in addition to inactivating undesirable microorganisms in order to extend storage life of foods. These processes will be discussed later in Lesson 6.
- Microorganisms, when added to food systems, to produce fermented foods, are essentially sources of desirable enzymes required to catalyse the desired chemical reactions needed to produce fermented food products (yogurt, sauerkraut, soy sauce). This will be discussed in more detail in Lesson 9.
- Enzymes are also extracted from a variety of sources (plants, animal by-products, microorganisms) and purified for use as aids in food processing (e.g. proteases used for milk coagulation during cheese making; pectinases to enhance juice recovery and for clarification of apple juice; invertase for conversion of sucrose to invert sugar; isomerase to produce high fructose corn syrup).
Food Proteins and Food Allergies
What is the relation between food proteins and allergies?
- Intolerance to certain proteins in foods is the basis for many food allergies. You will often see statements on labels for ice cream, cereals and candy bars that warn of the possibility that the product may contain traces of peanuts or other nut products.
- Allergies to peanut protein can be very severe, such that even with proper cleaning and sanitation, all residues of peanut allergens cannot be removed from processing equipment. Thus products that do not contain nuts, but that have been processed with equipment that was used for nut containing products may contribute enough allergen to cause some problems in very sensitive individuals.
- For recent food recalls in Canada due to potential allergy risks, see http://www.inspection.gc.ca/english/corpaffr/recarapp/recaltoce.shtml
Whant to learn more? |
|
2.2.1.4 Water
Terms to remember |
|
Water is an extremely important component of food systems. Water is related to all aspects of food ranging from our perception of quality, to the ability of microorganisms to be metabolically active in food systems.
Water exists in food in two forms: Free water and Bound water.
Free water
- Some water may be present within intergranular spaces, within pores of the food matrix and as a thin film of water on the surface of many foods.
- Free water can be found in tissue food systems and in dispersions.
- Water that is free and not bound by food components generally retains its usual physical properties, can also function as a dispersing agent for colloidal substances, can function as a solvent and can be used by microorganisms.
Bound water
- Some water can be adsorbed on surfaces of macromolecules such as starches, pectins, proteins through forces such as van der Waals forces and hydrogen bond formation.
- This water does not display all of its normal physical properties and it is not readily available for use by microorganisms and chemical or enzymatic reactions.
- Another form of bound water is the water that is associated with food matrices as water of hydration. That water is also not readily available for use by microorganisms and enzymatic and chemical reactions in the food matrix, whether it is a tissue based food system or a dispersion.
- Sugars and salts (sodium chloride) can bind substantial amounts of water and are often added to foods for the purpose of decreasing the amount of free water in the food system. In that context, sugars and salts can be used to control or prevent growth of certain microorganisms in foods.
What does water activity (aw) mean?
Water activity is a measurement that is frequently used in monitoring the availability of water (free water) in foods for the support of:
- microbial growth
- chemical reactions
- enzymatic reactions
Water activity can be measured as the ratio of the vapour pressure of water in the food to the vapour pressure of pure water, both measured at the same temperature.
- Aw = (Vapour Pressure of Water in Food at X °C) / (Vapour Pressure of Pure Water at X °C)
Please watch the following animation that depicts the effect of solutes on vapour pressure and water activity
Water activity can range from 0 (no free water) to 1.0 (all the water is free, such as in distilled water).
Water activity values of a number of food products are shown below. Water activity of foods can be adjusted by physically removing water from foods during concentration and dehydration processing operations, also when the water in the food is frozen (the free water is in a solid state, in the form of ice crystals), or by adding substances that bind water thus lowering the proportion of water in the free form. The most commonly used water-binding agents are sugars and salt.
Water activity of selected foods:
| Food | Water activity | Water content (%) |
|---|---|---|
| pure water | 1.00 | 100 |
| fresh meat | 0.97 | 65 |
| eggs | 0.97 | 75 |
| fruits, vegetables | 0.97 | 90 to 95 |
| bread | 0.96 | 35 |
| Cheddar cheese | 0.96 | 40 |
| frankfurters | 0.93 | 56 |
| salami | 0.90 | 61 |
| jams and jellies | 0.80 to 0.95 | 32 |
| honey | 0.75 | 18 |
| dried fruit | 0.60 to 0.70 | 20 |
| wheat flour | 0.70 | 12 |
Is water activity the same as moisture content?
The answer is no. Measurement of the moisture content of a variety of food systems is a frequently conducted quality assurance measurement. However, measurement of water content of foods does not indicate whether the water is bound or free.
The data in the above table shows that water content of foods cannot be used as a reliable indicator of the water activity.
2.2.2 Food Minor Components
Terms to remember |
|
A number of other food constituents are present in foods in much smaller quantities than the major constituents (carbohydrates, proteins, fats). These minor constituents are very important in their influence on our perception of the quality attributes of the food. The minor constituents are: organic acids, pigments and aroma compounds.
2.2.2.1 Organic Acids
Fruits contain natural acids which give the fruits tartness and slow down bacterial spoilage. Organic acids also impart flavour and acidity to food. Examples of organic acids and foods in which they are occur are:
| Organic acid | Food |
|---|---|
| Malic acid | apples |
| citric acid | citrus fruits, tomatoes, strawberries. |
| Tartaric acid | grapes |
| lactic acid | yogurt, cheese, olives, cottage cheese, sauerkraut. |
- The major uses of organic acids are to adjust pH or to acidify food, and to impart flavour. For example, acetic acid provides flavour and decreases pH; phosphoric acid provides flavour and tartness in beverages.
- A number of organic acids are also employed as antimicrobial agents.
- Organic acids have a wide range of textural effects in food systems due to their reactions with proteins, starches, pectins, and other food constituents.
- What is pH?
- pH is a measure of the acidity of a food. Foods and beverages differ in pH because of their content of acids, which produce hydrogen ions (Table 2.3). We are able to detect these ions by using a hydrogen sensitive electrode in a device called a pH meter.
Table 2.3. Average pH values and acidity classification of selected foods.
| Chemicals Contributing to Aroma of Coffee | pH value | Acidity classification |
|---|---|---|
| Meat, fish, poultry | 7.0 | Low acid
(pH > 4.6) |
| Milk | 6.5 | |
| Corn | 6.3 | |
| Wheat flour | 6.0 | |
| Potatoes, peas | 5.8 | |
| Carrots | 5.1 | |
| Figs | 5.0 | |
| Apples | 3.7 | Acid
(pH 0 - 4.6) |
| Cherries | 3.6 | |
| Oranges, pears, tomatoes | 3.5 | |
| Pickles | 3.0 | |
| Lemon/lime juice | 2.3 |
Is pH important to the Food Industry?
Yes. An important pH for the food industry is pH 4.6; this is the borderline between an acidic food and a low acid food (Table 2.3).
- acid foods have pH of 4.6 or less
- low-acid foods have pH greater than 4.6
Acid foods will not support growth of disease causing microorganisms. This aspect will be discussed in more detail later in the course.
2.2.2.2 Colours and Pigments in Foods
Many food systems are coloured by pigments naturally present in the food system:
Chlorophyll, the green pigment in plants, is responsible for the green colour in apples, lettuce, celery and broccoli. Chlorophyll a has a blue green hue (e.g. in the florets of fresh broccoli) while chlorophyll b has a yellow green hue (stems of broccoli).
Carotenoids, a diverse group of pigments, can be subclassified into carotenes and xanthophylls. Carotenoids naturally produce red, orange and orange-yellow colours in many foods (e.g. tomatoes, carrots, pineapples, shrimp)
Anthocyanidins and anthocyanins (anthocyanidin complexed with glucose or other sugars). These pigments are the predominant colour pigments in blueberries, cherries, cranberries, plums and red cabbage. The anthocyanins are particularly sensitive to changes in pH, showing marked changes in colour with pH changes in the food system. See Figure below:
As you can see in the above figure, the colour of an athocyanin is most stable and most highly coloured at low pH values. The colour will be gradually lost as the pH increases. Notice that at pH 5, the anthocyanin is almost colourless. The colour loss is reversible, and the red hue will return upon acidification.
Other pigments in food systems include hemoglobin and myoglobin (the red pigments in blood and muscle).
All of the pigments noted above are sensitive to varying degrees to changes in the environment (pH, presence or absence of oxygen, presence of metal ions, enzymatic degradation) in the food system. Thus colour changes often occur in fresh fruits, vegetables, meats and fish during storage, spoilage and as a result of processing and cooking.
In addition, there are a number of pigments, either those extracted from plants or microorganisms, or synthetic pigments that are used as colouring agents in fabricated food systems. These will be discussed in more detail in the section on food additives (Lesson 4).
2.2.2.3 Aroma Constituents
The aroma profile of foods is very complex. The aroma of food is detected when we inhale volatile constituents of foods that react with the receptors in the olfactory regions of our nasal passages.
Most of us have experienced the aroma of freshly brewed coffee; there are actually hundreds of volatile compounds that have been identified in the aroma. The table below is a list of some of the chemical components that have been identified to contribute to the aroma of coffee; you don't have to memorise any of these compounds, it is only an example to demonstrate the amount of constituents that influence the aroma we perceive on a food.
| Chemicals Contributing to Aroma of Coffee | |
|---|---|
| Acetaldehyde | Hydrogen sulfide |
| Acetic acid | Hydroquinone |
| Acetone | Isovaleric acids |
| Acetyl methyl carbinol | Methyl alcohol |
| Ammonia | Methyl amine |
| Cresols | Methyl ethyl acetic acid |
| Diacetyl | N- methyl pyrrole |
| Diethyl ketone | p-Vinyl guaiacol |
| Dimethyl sulfide | Phenol |
| Esters | Pyrazine |
| Ethyl alcohol | Pyridine and homologues |
| Formic acid | Resorcinol |
| Furane | Sylvestrine |
| Furfural | Trimethylamine |
| Furfuryl alcohol | Vanillone |
| Guaiacol | |
| Higher fatty acids | |
Adapted from: Benarde, M.A. 1971. Chemicals We Eat. American Heritage Press, N.Y., pp.208.
Please note that:
- No single chemical compound can be attributed as being the sole source of the aroma of a particular food product.
- It is the specific mixture of chemicals in a particular concentration that creates the aroma that we associate with a high quality food product.
- Any change in that specific mixture of volatile compounds or their concentrations will alter the aroma that we perceive.
- The volatile constituents that contribute to the aroma of foods are present in very low concentrations but are nonetheless very important constituents of foods.
- The flavour constituents are either present as part of the food matrix (fresh strawberries) or are modified (cooking of strawberries) or created (roasting of coffee) during processing or cooking.
2.2.2.4 Vitamins and Minerals
Vitamins are mainly classified into water-soluble and fat-soluble vitamins. Vitamins are organic compounds that make up a small portion of food; however, vitamins are very important from a nutritional point of view because they are essential components of the human diet as they carry out some very important tasks in the body.
- Water-soluble vitamins include vitamin C (ascorbic acid), thiamin, riboflavin, niacin, pyridoxine, vitamin B12, and folacin. These vitamins are found within the water (aqueous) phase of foods.
- Fat-soluble vitamins (vitamin A, vitamin D, vitamin E) are found within the fat (oil) portion of foods
Although Vitamins do not contribute to the physical characteristics of food, some are actually used as "food additives". Below are some examples of the two most common vitamins used as food additives:
| Vitamin | Food Additive category | Function |
|---|---|---|
| Ascorbic acid (Vitamin C) | Bleaching agents | *Hasten oxidation and aging processes as in flour whitening treatment |
| Ascorbic acid (Vitamin C) | Preservatives | Act as antioxidant to slow down rancidity and browning reactions |
| Tocopherols (Vitamin E) | Preservatives | Antioxidant |
* Ascorbic acid converts to its oxidizing from during mixing
Minerals (calcium, magnesium, sodium, potassium, iron, zinc) are often associated with various cellular components in tissue food systems and often are active participants in chemical and biochemical reactions that affect the chemical properties and textural characteristics of food systems.