Course:FNH200/Lessons/Lesson 02/Page 02.1
2.1 Food-Colloidial Dispersions
Terms to remember |
|
Foods are essentially mixtures of chemical compounds arranged in specific organizations that give rise to the particular chemical, physical and sensory properties of each food system. A knowledge of the chemical and physical properties of the constituents of foods is important to your understanding of how food systems behave under the various conditions encountered in preservation, storage and preparation for consumption. Food systems vary in their chemical composition and physical properties, ranging from chemically simple systems such as sugar syrups to the chemically complex food systems such as milk, muscle food systems, and plant tissue systems.
In Colloidal Dispersions, the particles of one substance are distributed, dispersed, in another substance without dissolving.
- The substance that is dispersed within another is called the dispersed phase.
- The substance that extends throughout the system and surrounds the dispersed phase is called the continuous phase.
Table 2.1 shows the dispersed and continuous phases of some typical Colloidal dispersions.
Table 2.1. Colloidal dispersions
| Dispersed Phase | Continuous Phase | Name of Dispersion | Examples |
|---|---|---|---|
| solid | liquid | sol | starches, proteins and some plant polysaccharides in water |
| liquid | liquid | emulsion | milk, mayonnaise |
| liquid | solid | solid emulsion | butter, margarine |
| [liquid] | solid | gel | starch, pectin or gelatin gels |
| gas | liquid | foam | beaten egg white, whipped cake frostings |
| gas | solid | solid foam | meringue, ice cream, bread |
For example, a sol is a suspension of large molecules dispersed in a liquid, generally water.
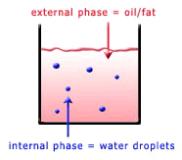
An emulsion is a suspension of liquid droplets (fat or water) within a liquid medium (fat or water). Food emulsions can be either oil in water (o/w) or water in oil (w/o). Homogenized milk is a dispersion of milk fat droplets in a liquid medium (skim milk portion of milk), while skim milk itself is a suspension of milk protein particles, the casein micelles, within a water-based medium. A solid emulsion is a dispersion of liquid droplets within a solid phase. Margarine and butter are examples of water in oil emulsions, in which the continuous phase is solid under refrigerator or low ambient temperatures.
A gel is a dispersion of water held within a continuous matrix of polysaccharides (starch gels) or proteins (gelatin gels). Some scientists consider the water in gels to be a second continuous phase rather than a dispersed phase.