Course:EOSC311/2022/Carbon as a resource
Carbon is an essential resource to all known lifeforms, Its ability to form bonds with other atoms to create biomolecules like DNA. By this fact alone, it is required by any organisms that are expected to grow and reproduce. Through billions of years, carbon on earth has taken many forms. Some contribute to form living organisms, while others become minerals or parts of the atmosphere. Carbons can control the ability for plants to respire, the climate, and to provide the recourses that allow mankind to operate in modern society. This wiki page will explore the many forms that carbons take on during the history of the earth, how they became a resource and the repercussions of such usage.
Statement of connection and why you chose it
I believe that we must make the most of all the resources we have been given. However, through my studies in the Natural Resources Conservation major, we learned about the consequences of using such resources. Fossil fuels is in many ways an amazing resource, it has advanced human science, technology and medicine in extraordinary ways, despite their side effects. Most people would consider it a worthwhile trade-off (for now). Throughout my degree, I hope to learn more about these resources; discovering the geological processes that create them, how their use is harmful to humans and the natural world and how we should manage these resources. Conservation may be the name of my program but I want to explore and understand more about the creation and the usage of such wonderful resources.
The Movement of Carbon
The Long-Term Carbon Cycle
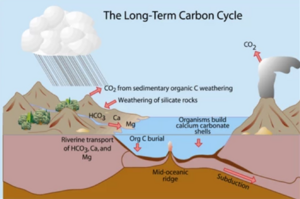
The long-term carbon cycle involves the moment amongst the layers within the earth, moving carbon through the lithosphere every the course of millions of years. Much of the Earth's carbon is stored through this processed. The lithosphere stores about 99.9% of all carbon on earth, in the form of minerals, crystals and fossil fuels. Other carbon sinks and storages, such as in the biosphere, oceans, and the atmosphere only contain as little as 0.1% of Earth's carbon at any given time (Gervais, 2015). However, during the long-term carbon cycle, carbon moves through these spheres back into the lithosphere through processes like; weathering, erosion, burial and photosynthetic respiration. While the carbons from the lithosphere are released back into the other spheres through volcanic activity and the burning of fossil fuels.
These long-term cycle begins with the diffusion of atmospheric co2 from the atmosphere into the ocean, which is then utilized by marine organisms to fix the dissolved carbon into their shells and skeletons (calcium carbonate). Through the decomposition of these organisms, the calcium carbonate will sink and accumulate at the bottom of the ocean. With enough heat and pressure, carbon-rich sedimentary rocks are created. these rocks are then moved by the ocean plates and subducted into plate boundaries and into the mantle. Then the cycle will continue when the volcanoes that are formed at the plate boundaries erupt, releasing the carbon back into the atmosphere (Gervais, 2015).
Weathering
Weathering is the process when solid rock is dissolved or broken apart into smaller fragments or particles. Chemical weathering is a type of weathering that changes minerals in rocks through chemical reactions involving water. In the context of the carbon cycle, when carbon dioxide is mixed with rainwater in the atmosphere, carbonic acid is created. This weak acid works to slowly dissolves rock material that it flows through, releasing calcium ions that are carried into bodies of water and eventually into oceans. These calcium ions will combine with bicarbonate ions in the seawater to form calcium carbonate sediments. The calcium carbonate sediments are crucial to many organisms in the ocean, namely coal and shellfishes. These organisms utilize calcium carbonate ions to build their shells. When these organisms die, their shells and the remnants of their body that weren’t decomposed will cement together and form carbonate rocks, such as limestone, accumulating into immense storages of carbon over time, locking them in long-term storage in the lithosphere.
Photosynthesis
Another way that carbon can be locked into long-term storage in the lithosphere is through photosynthetic organisms. Through the processes of photosynthesis, these organisms convert solar and carbon dioxide from the atmosphere into energy and carbon in their body. Under aerobic conditions these organisms will decompose after they die, recombining their carbon with the oxygen in the air to form carbon dioxide again. However, in environments with the absence of oxygen, the carbon molecules cannot recombine to form carbon dioxide again. Instead, its tissue will be preserved rather than decomposed. The accumulation of remains preserved in marine sediments or in peat wetlands becomes a part of the lithosphere over millions of years, eventually, they will become fossil fuels.
The Short-Term Carbon Cycle
The more commonly discussed carbon cycle is likely the short-term carbon cycle involves the movement of carbon amongst oceans, the atmosphere and the biosphere. This cycle occurs in a time scale that can be more influenced by humans, ranging from a few minutes to thousands of years. Much of this cycle is also driven by photosynthesis, where photosynthetic plants or algae intake carbon dioxide from the atmosphere. The decomposition of these organisms or the organisms that directly feed on them will release carbon back into the atmosphere, repeating the cycle.
History of Climate Change
The history of Earth's atmospheric carbon dioxide concentration has been determined by scientists drilling out ice cores from the ice sheets of Greenland and the Antarctic and carefully analyzing them for ancient gas bubbles that were preserved in the ice. These ancient gas bubbles are able to provide a snapshot of what the atmosphere would have been like at the time that it was frozen (Jayawardena, 2015).

As observed from Figure 2. the natural atmospheric carbon dioxide concentrations have fluctuated greatly in the last 800000 years. The troughs of the graph represent Ice ages where more carbon is trapped in glaciers. Due to factors like the Milankovitch cycles, where the tilt and the elliptical-shaped orbit of the earth around the sun (Lindsey, 2020). When the earth is at the farthest point in the elliptical orbit, an ice age occurs as the temperatures drop significantly (Lindsey, 2020). This drop in temperate not only traps carbon in ice, much of flora and fauna are also not able to grow at this time, thus hindering the carbon cycle from progressing. However, when points of the orbit where the earth is much closer to the sun, a warming period occurs melting glaciers and releasing all of the previously trapped carbon back into the cycle, in addition to the global temperature becoming more suitable for organisms to survive in. These are the peaks of carbon dioxide in Figure 2.
Anthropogenic Contributions
Since the beginning of the Industrial Revolution in the 1700s humans have been burning fossil fuels for energy, accelerating the movement of carbon from the lithosphere into the atmosphere, disrupting the delicate natural self-regulating cycle. Prior to the industrial revolution, the main way carbon is released back into the atmosphere in the carbon cycle is through volcanic activity. However, as Gervais explains, modern society releases about 100 - 300 times more carbon into the atmosphere through the burning of fossil fuels than all of the world's volcanic activity combined (2015). Fossil fuels are a natural part of the long-term carbon cycle, but they are now being transferred from the long-term storage of the long-term carbon cycle to the short-term one.
Our Current Atmosphere
Our current atmosphere has the highest concentration of carbon dioxide in recorded history. As illustrated by Figure 2. The previous highest atmospheric carbon dioxide concentration occurred about 300000 years ago at 300 parts per million. Currently, as recorded in 2020 the carbon dioxide concentration in our atmosphere is about 412.5 parts per million in 2020, and about 418 ppm as measured in May 2022 (NASA, 2022). No other time in the recorded history of our planet has released as much carbon dioxide into our atmosphere as today.
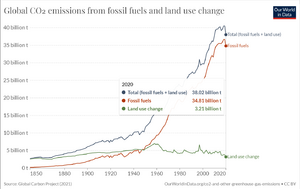
As illustrated in Figure 4. human activity contributes about 38 billion tons of carbon dioxide into the atmosphere per year, through a combination of deforestation, and using fossil fuels as a resource. These factors culminate to about an entire year's worth of volcanic carbon dioxide emissions in just 4 days (IPCC Report, 2014).
Repercussions
Carbon dioxide in our atmosphere acts as a greenhouse gas, trapping heat that’s reflected off the surface of the earth by the sun. The more abundant greenhouse gases we have in our atmosphere the more heat is trapped. More worrisomely, the accelerated heating of the Earth will not occur linearly and could be quite unpredictable. There are factors such as the ice-albedo feedback loop that are not self-regulating. Where the rate of glacial melt is accelerated when any of it is melted. Albedo refers to the ability of an object to reflect solar insolation away from itself instead of absorbing its energy because snow and ice are very shiny light in colour their capacity to reflect incoming solar insolation is very high, thus it is considered high albedo. However, once this ice and snow melts, it reveals the ground underneath, usually dirt, and often very matte and very dark. Dirt's ability to reflect solar insolation is not nearly as good as snow or ice. Dirt absorbs this energy instead, turning much of it into heat. This heat is then transferred to its surrounding areas resulting in the melting of other snow and ice. Another factor comes from the soot that is released by the combustion of fossil fuels, these dark particulates could land on snowy and icy surfaces and decrease their albedo, thus accelerating their warming (Gervais, 2015). NASA claims that the arctic is already warming at about twice the global rate (n.d).
Ecological Damage
Flora:
We have witnessed a significant increase in wildfire intensity and frequency over the last century. Due to the increased temperature, and evaporation (Abatzoglou et al., 2018). The burning of forests also contributes to climate change in a positive feedback loop. Since forests act as carbon sinks, the destruction of which would hinder sequestration of carbon dioxide in the atmosphere, leading to warmer environments which leads to more fires. In addition to the increased risk of fire, our forests are also becoming increasingly vulnerable to pests, such as the mountain pine beetle. Previously, the propagation of these pests would be controlled by the winter seasons, where much of them would die off due to the cold. Now that the warming capacity of our planet has increased through the abundance of greenhouse gasses and the accelerated melting of glaciers, the winter season are becoming warmer, resulting in more pests surviving (Kurz et al., 2008). The mountain pine beetle is one of such notorious pests in North America. In Canada alone, approximately 723 million cubic metres of merchantable pine have been lost to the mountain pine beetles (Government of Canada, 2021). Akin to the forest fires pest outbreaks that lead to the destruction of trees and their ability to function as carbon sinks also become a positive feedback loop.
Fauna:
In addition to forests, climate change has also affected many other important organisms that may not be able to adapt quickly enough. The Pacific Salmon is one such organism. They provide ecosystem services including, provisioning, supporting and cultural services to the Fraser river ecosystem. The warming of the area due to climate change has had many devastating effects on the species. The increased temperature is correlated with increased adult pre-spawn mortality and sickness, diminish egg viability, and has legacy effects that harm juvenile health (Grant, 2019). Salmon that move to their spawning grounds during the summer months are more stressed and have fewer energy reserves, which has a negative impact on swim performance and survival. Salmon can die before reaching spawning grounds due to high temperatures in the river. Changes in precipitation, such as dryness followed by heavy rain, can cause landslides, which can harm freshwater habitats (Grant, 2019). These factors not only are devastating to the local natural ecosystem but also to the communities of people who depend on them for survival.
Conclusion / Your Evaluation of the Connections
It is clear that the usage of carbon in the form of fossil fuels to fuel our society comes at a great cost. The scale and complexity of the Long-Term carbon cycle are almost difficult for humans to imagine. Recently in our course, we learned about the creation of diamonds, another incredible carbon-based resource. Its creation process is similar to fossil fuels, both are made from carbon and requires immense pressure and temperatures over the course of millions or billions of year to materialize. However, with modern technology diamonds could be synthetically grown in labs with similar properties to natural ones. I am curious as to the possibility of synthetic fossil fuels. That still doesn’t address the problems of carbon emissions through their usage though. In this wiki page, we have explored the concept of carbon sinks, such as the oceans and forests, where the oceans consume carbon dioxide by dissolving into seawater and eventually becoming sedimentary rocks. As I learn more about geology, I hope to learn more about this process and explore the possibilities of creating this process ourselves. If we could match the accelerated release of carbon but equally match the deposition of it back into the earth much of the discussed side effects would be less severe.
References
Abatzoglou, J. T., Williams, A. P., & Barbero, R. (2019). Global emergence of anthropogenic climate change in fire weather indices. Geophysical Research Letters, 46(1), 326–336. https://doi.org/10.1029/2018gl080959
Canada, N. R. (2021, April 28). Government of Canada. Natural Resources Canada. Retrieved June 22, 2022, from https://www.nrcan.gc.ca/forests/fire-insects-disturbances/top-insects/13397
EarthLabs. (2021, November 19). 3B: CO2 - my life's story. Climate and the Carbon Cycle. Retrieved June 22, 2022, from https://serc.carleton.edu/eslabs/carbon/3b.html
Gervais, B. (2015). Living Physical Geography. CALIFORNIA STATE UNIVERSITY.
Grant, S. C. H., MacDonald, B. L., & Winston, M. L. (2019). (rep.). State of Canadian Pacific Salmon: Responses to Changing Climate and Habitats. Nanaimo, British Columbia : Fisheries and Oceans Canada.
IPCC, 2014: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC, Geneva, Switzerland
Jayawardena, A. W. (2015). Climate change — is it the cause or the effect? KSCE Journal of Civil Engineering, 19(2), 359–365. https://doi.org/10.1007/s12205-015-0524-8
Kurz, W. A., Dymond, C. C., Stinson, G., Rampley, G. J., Neilson, E. T., Carroll, A. L., Ebata, T., & Safranyik, L. (2008). Mountain Pine Beetle and forest carbon feedback to climate change. Nature, 452(7190), 987–990. https://doi.org/10.1038/nature06777
Lindsey, R. (2020, August 14). Climate change: Atmospheric carbon dioxide. Climate Change: Atmospheric Carbon Dioxide | NOAA Climate.gov. Retrieved June 22, 2022, from https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide
NASA. (2022, March 15). Carbon dioxide concentration. GLOBAL CLIAMTE CHANGE Vital Signs of the Planet. Retrieved June 22, 2022, from https://climate.nasa.gov/vital-signs/carbon-dioxide/
Ritchie, H., & Roser, M. (2020, May 11). CO2 emissions. Our World in Data. Retrieved June 22, 2022, from https://ourworldindata.org/co2-emissions#citation
| This Earth Science resource was created by Course:EOSC311. |
