LFS:SoilWeb/Soil Biology/Nutrient Cycles/Phosphorus (P)
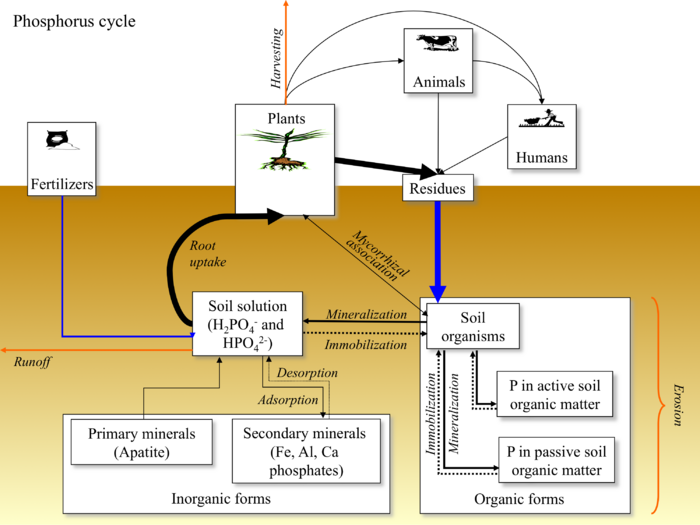
Phosphorus Cycle
The most important plant function associated with P is the storage and transfer of energy. The energy obtained from photosynthesis and metabolism of carbohydrates is stored in phosphate compounds vital for plant growth, such as ADP or ATP (adenosine di- and triphosphates). Synthesized through both respiration and photosynthesis, ATP contains high-energy phosphate groups that drive most energy-requiring biochemical processes.
P is also an essential component of DNA (deoxyribonucleic acid), the seat of genetic inheritance, and of RNA (ribonucleic acid), which directs protein synthesis in both plants and animals.
Phosphorus is also part of phospholipids, which play critical roles in cellular membranes.
P is most commonly in the form of phosphoric acid (H3PO4) in soil, but it is taken up most readily in the form of phosphate ions (H2PO4- and HPO42-). P is limited in most soils because it is released very slowly from insoluble phosphates. Under most environmental conditions it is the limiting element because of its low concentrations in soil and high demand by plants and microorganisms. Many plants have increased their P uptake by adapting a symbiotic relationship with mycorrhizae. A P deficiency in plants is characterized by an intense green colouration in leaves. If the plant is experiencing high P deficiencies the leaves may show signs of necrosis or occasionally the leaves may appear purple. Because P is a mobile nutrient, older leaves will show the first signs of deficiency.
Sources
Phosphorus Fertilizers
Phosphate fertilizers come from rock phosphate, i.e. apatite [3Ca3(PO4)2] CaF2. The rock is either heat-treated or acid-treated to break the apatite bond and to render the contained phosphate more soluble. Acid-treated phosphate is the most common type of phosphorus fertilizer.
Examples of acid-treated phosphates include: phosphoric acids (H3PO4), superphosphates, ammonium phosphates, ammonium polyphosphates, and nitric phosphates.
Animals
Animals consume plants and in turn add various residues (excrement and remains) to the soil that then serve as a source of soil organic matter or humus.
Humans
Humans consume plants and in turn add various residues to the soil that then serve as a source of soil organic matter or humus.
Transformations
Mineralization
Mineralization is the overall process of conversion of phosphorus from an organic to an inorganic form as a result of microbial decomposition. The inorganic phosphorus released by mineralization is readily available to higher plants and to microorganisms.
Immobilization
Immobilization is the process of conversion of phosphorus from the inorganic (mineral) form to the organic form in microbial or plant tissues, thus rendering phosphorus not readily available to other organisms or plants.
Phosphate Adsorption (Fixation)
The ionic forms of P (i.e., phosphate ions) are susceptible to adsorption and subsequent precipitation either by Fe, Al and Mn present in the soil solution (in acidic soils) or by Ca compounds (in alkaline soils). At neutral pH, phosphates tend to bind with clay minerals (i.e., 1:1 type minerals and accompanying hydrated oxides of Fe and Al). Phosphate compounds with either Fe, Al, Mn, or Ca tend to have low solubility and consequently low availability to plants. This is referred to as phosphate fixation to indicate that phosphorus is tied up in a form unavailable for uptake by plants.
Example of phosphate fixation by aluminum ion:
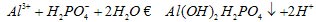
The highest concentration of dissolved phosphates in soil solution is at pH of about 6 to 7.
Losses
Harvesting
Removal of plant residues is the main pathway of losing phosphorus from the soil. Annually, about 5 - 50 kg P/ha are removed in harvested biomass.
Erosion
Erosion is one of the main pathways through which phosphorus is lost from the soil. It is estimated that between 0.1 and 10 kg/ha annually are lost by erosion of P-carrying soil particles.
Runoff
Runoff is one of the main pathways through which phosphorus is lost from the soil. It is estimated that between 0.01 and 3 kg/ha annually are lost as phosphorus dissolved in runoff water.
Forms of P
Soil Solution
Phosphorus is absorbed by plants as the primary and secondary orthophosphate ions (H2PO4- and HPO42-), which are present in the soil solution. The amount of each form present depends on soil solution pH. At pH = 7, both phosphate ions are present in about equal amounts. At pH < 7, H2PO4- is the main form, while at pH > 7, HPO42- dominates.
Primary Mineral
The native phosphorus in soils originated largely from the weathering of rocks containing mineral apatite Ca10(PO4)6(F, Cl, OH)2. Phosphorus present in this form is non-labile, i.e. unavailable to plants.
Secondary Minerals
Secondary minerals of phosphorus include Fe and Al phosphates and Ca phosphates that are very insoluble and as such, not available to plants. Phosphorus present in the soil in secondary minerals is non-labile (unavailable).
Organic Forms of Phosphorus
Three groups of organic phosphorus compounds exist in soils:
- Inositol phosphates (phosphate esters of a sugar-like compound inositol C6H6(OH)6 can accumulate and account for more than half of the organic phosphorus and 1/4 of total phosphorus in soils.
- Phospholipids (esters of fatty acids) are derivatives of glycerol. The rate of release of phospholipids from organic sources in soils is rapid.
- Nucleic acids - There are two distinct forms of nucleic acids in all living things: RNA (ribonucleic acid) and DNA (deoxyribonucleic acid). They are released into soil and broken down more rapidly than the inositol phosphates.
Much of the remainder of organic phosphorus in soils is thought to originate from microorganisms, especially from bacterial cell walls.
Mycorrhizal Associations
Mycorrhizae (means "fungus root" in Greek) is the symbiotic association between fungi and the root of a plant. The hyphae of symbiotic mycorrhizal fungi are beneficial to the plant in soils where phosphorus diffusion is slow, because phosphorus is transported inside hyphae by cytoplasmic streaming, making the plant much less dependent on the diffusion of phosphate ions through the soil.