SPPH381B/Essays/TermProject/Aluminum Foil - Soham/Bayer Process
Bayer Process
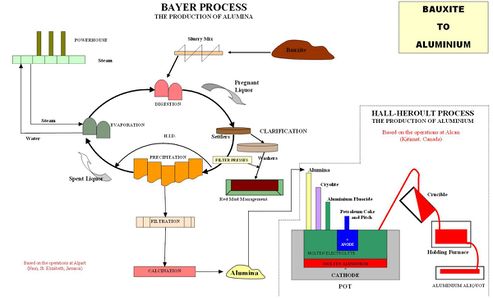
The first step in purifying the mined aluminum ore is to remove any other metal oxides that may be mixed with the impure aluminum. This involves the Bayer process, where bauxite is smashed and mixed with sodium hydroxide. Next, the mixture is shuttled to pressurized tanks, where the bauxite mixture is further broken down into sodium aluminate.
Afterwards, the mixture is clarified through various tank and press machines, where the sodium aluminate is filtered further. The aluminum oxide mixture is then precipitated in a silo to cause aluminum particles to form. Finally, the solution is calcified by heat and starts to form aluminum oxide powder. This involves dissolving the oxide in a mold filled with carbon and a heated liquid conductor, followed by running a current through the cell. The product is a molten form of aluminum, which is transferred to a furnace to be heated to a liquid form and then cooled to be rolled. [1]
If bauxite contains more than 10% silica, due to economic reasons, the Hall-Heroult process is used to purify bauxite. [2]
During the smashing and mixing (Bayer Process) of the primary manufacturing of bauxite, low-level radioactivity present in bauxite may be a minimal concern. This radioactivity could potentially have mutagenic effects on the human body. Although bauxite dust from crushing resulted in only a single case of Pulmonary Fibrosus in a case report, it is not correlated with clinically significant declines in lung disease or function. Contamination of cooling water towers may be a potential biological hazard, along with infectious disease and animal encounters in tropical countries where bauxite refining may occur. Bauxite dust exposure was not significantly associated with an increased cancer risk of any type. Chemical splashes are common in refineries due to various chemicals such as caustic soda being used. Physically, confined spaces presents dangers of a hypoxic, flammable or toxic environment in alumina refineries. [3]
Summary of Hazards [3]
Physical Hazards
1. Heavy equipment used in the process of aluminum refining can trap limbs and fingers of workers.
2. Loud noise caused by heavy machinery.
3. Bauxite ore is known to present low-level radioactivity through Uranium, Potassium, and Thorium.
Ergonomic Hazards
1. Repetitive motions required to operate refinery machines may lead to musculoskeletal disease.
Chemical Hazards
1. Chemical burns from caustic by-products and use of sodium hydroxide can occur.
2. Small fibres of aluminum dust can accumulate in lungs and cause malignancies, although this is very rare.
3. Noncondensable gases containing volatile organic compounds can be produced during the digestion phase of the Bayer Process from the breakdown of organic compounds.
Biological Hazards
1. Contamination of cooling water towers used during the Bayer process can lead to spread of infectious disease such as malaria in tropical countries.
Safety Hazards
1. Confined spaces present a significant risk in aluminum refineries where toxic and highly flammable gases can accumulate.
References
- ↑ Madehow.com. (n.d.). Aluminum Foil. Retrieved March 27, 2017, from <http://www.madehow.com/Volume-1/Aluminum-Foil.html>.
- ↑ ACS.org. (n.d.). Hall Process Production and Commercialization of Aluminum - National Historic Chemical Landmark. Retrieved from <https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/aluminumprocess.html>.
- ↑ 3.0 3.1 Donoghue, A. M., Frisch, N., & Olney, D. (2014). Bauxite Mining and Alumina Refining: Process Description and Occupational Health Risks. Journal of Occupational and Environmental Medicine, 56(5 Suppl), S12–S17. http://doi.org/10.1097/JOM.0000000000000001>.