Evolution of Echolocation in Bats
The Origin of Echolocation and Phylogeny in Bats
Fossil records from the Eocene Epoch (56 to 34 Mya) are the earliest known existence of 'completely developed' bats, which were preserved in their entirely, to suggest that echolocation was developed very early in their evolution [1]. It was a period in time where Earth must have been almost completely covered in forest due to warm climates, creating a rich flora of tropical plant species and marked the emergence of the first modern mammals [2]. These fossilized bats were already capable of powered flight and echolocation. However, because of the lack of any prior fossil evidence, it was difficult to explain their evolution by natural selection, and it was commonly used to support theories of irreducible complexity in that past [3].
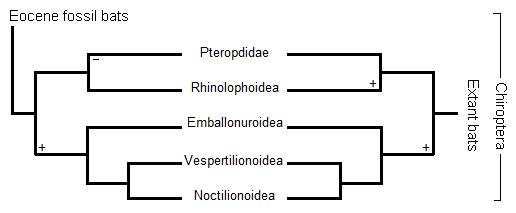
Traditionally, the phylogeny of bats is considered to be split into two monophyletic suborders; Megachiroptera (megabats) and Microchiroptera (microbats) based solely on morphological and paleontological data. Only microbats are capable of using laryngeal echolocation (calls produced in the larynx), with the exception of one genus of megabats, Rousettus (who use lingual echolocation). [4]. It is believed that the single common ancestor of mega- and microbats was already able to perform echolocation, but later on megabats subsequently lost the ability[5].
Increasing molecular studies however, using bootstrap and maximum likelihood analyses have weakened the monophylogeny of bats and given support to two new suborders; Yinpterochiroptera (all non-echolocating bats and one microbat superfamily) and Yangochiroptera (the remaining echolocating bats) to create paraphyly within the bat species to suggest that echolocation may have evolved multiple times [6].
Yinpterochiroptera includes Pteropodidae (megabat family) and the mircobat superfamily Rhinolophidae.
Yangochiroptera includes the remaining microbat superfamilies; Emballonnuroidea, Vespertilionoidea, and Noctilionoidea [7].
Figure 1. The relationship between extinct and extant superfamilies of bats displaying the two possible ways echolocation may have evolved. On the left it is showing echolocation deriving from a common ancestor and presumably becoming lost in Pteropodidae. On the right it shows echolocation evolving twice [8].
Two echolocation genes have been discovered; FOXP2, the 'vocalization' gene, and Prestin, the 'hearing' gene. However, the two conflict and cause the true evolution of bats to remain cryptic to cause the gene and species trees to also greatly differ. They are controversial because each molecular study supports a different hypothesis about the rise of echolocation in bats. The FOXP2 favours a common ancestor and either the loss or independent gain of echolocation in Pteropodids. While the Prestin gene favours convergent evolution in echolocating species[9]. The ambiguity of the evolutionary history of bats causes much debate and remains to be discovered[10]. However, one reason that may have caused this paraphyletic grouping in bats is because they are the only mammal to have evolved flight. Thus, bats that use echolocation and those that do not share many genes in common which are associated with flight and because no true 'echolocating' gene has been discovered, it may be the cause of any similarities shared between them [11]. The phylogeny of bats is believed to be clouded due to adaptive radiation of the species during the Eocene Epoch [12]. Models explaining the evolution of echolocation commonly began with the universal assumption that flight evolved first where Padian (1987) reasoned that ‘it [was] clear that the common ancestor (of bats)… was small, nocturnal, insectivorous and probably arboreal.’ However it has recently been thought that ancestral bats were really diurnal, frugivorous, and arboreal and later evolved to become nocturnal and insectivorous as Padian described. The spread of angiosperms (flowering plants) allowed for an ample supply of fresh fruit that could be exploited by small mammals such as bats. An increase in the diversity of insects also acted as a food source, and frugivorious bats may have eaten the occasional insect to prevent nitrogen deficiency. Thus, natural selection may have acted on the species to evolve to specialize in only consuming insects during the afternoon in which insects were the most active. The transition to nocturnality involved the increase risk of predation from raptorial birds as they also diversified. Flight at night then must have strongly selected from an increase in sensory function, causing echolocation to co-evolve with flight[13].
Echolocation Gene in Bats
The specific genes that bats use to allow laryngeal echolocation to occur have been hard to find by biologists. The reason for the limited number of findings is that the genes involved would depend on the abilities to make and direct the high frequency calls, to hear the high frequency returning echoes and to interpret these calls. [14] To date, only two potential echolocation genes have been studied thoroughly: FOXP2 and Prestin.
Vocalization Gene
FOXP2, considered the ‘vocalization gene’ of echolocation, is a transcription factor involved in the development and control of orofacial movements that allows the capabilities of vocalization in mammals. Mutations in FOXP2 cause both motor and comprehension-related speech impairment in humans. These mutation results explain why FOXP2 is a plausible gene for echolocation. In most mammals, FOXP2 was conserved, while when looked at in bats, the gene was highly variable, meaning it was subdue to change. [15]
When sequencing the FOXP2 was, two conflicting signals arise in Exon 17 and Exon 7. Exon17 shows high purifying selection in Ptreopodids and the FOXP2 gene being conserved, like in other mammals. However, Exon 7 shows that Ptreopodids have similar levels of non-synonymous variation and accelerated mutation rates commonly found in echolocating lineages. This evidence shows there were past echolocation capabilities in Pteropodids and there was a lost of the ability to echolocate. [16]
Hearing Gene
The gene Prestin is the most examined and best gene found that associates with the capability for bats to echolocate. Prestin, known as the ‘hearing gene’ of echolocation, detects and selects high frequency sounds for amplification as well as, shortens and stiffens the outer hair cells of the cochlea to make bats sensitive to the ultrasonic frequencies used in echolocation. [17] When exposed to high frequency sounds, Prestin changes in shape and causes the deformation in the cochlea hair cells that sends an electrical impulse to the brain. [18] Uniquely, Prestin converts voltage to force directly rather than driven by enzymes that require ATP-hydrolysis. [19] In addition, Prestin encodes a transmembrane motor protein which is used to drive the electromotility of the outer hair cells and amplifies the sound. [20]
The Prestin gene, unique to mammals, resulted from directional selection that acted on orthologues, since the split of mammals from a common ancestor with birds. [21] Further, positive selection acting on the Prestin gene occurred in bat species that used specialized constant-frequency echolocation that is associated with sharp tuning in the auditory system. [22] When sequencing the coding region of Prestin, shows that all bats that use laryngeal echolocation evolved similarities in the Prestin gene due to convergent evolution at the amino acid sites that are functionally important for echolocation. [23]
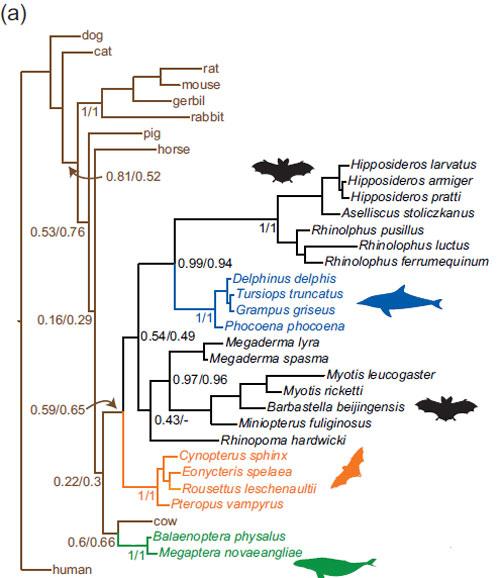
Conflicts
However, there are conflicts that arise of whether FOXP2 and Prestin are the true genes behind echolocation in bats. One of these conflicts is the comparison of the Prestin gene and the bat species phylogenetic trees. The phylogenetic tree that is base on the Prestin gene sequencing shows that bats that use the larynx to produce echolocation calls would form a monophyletic group in the phylogenetic tree. However, the phylogenetic tree of the history of bat species based on a large-scale genetic analysis of nuclear and mitochondrial DNA showed that laryngeal echolocators are paraphyletic. In addition, in the species phylogenetic tree, non-echolocating fruit bats are seen as a sister group to CF-bats that use sophisticated laryngeal echolocation. [24] This conflict in the pprestin gene relates to the evolutionary difference between the two echolocating genes. For the FOXP2 gene, a common ancestor is favored and the concept of divergent evolution that once enabled echolocation in Ptreopodids. However, in the Prestin gene, convergent evolution and positive selection is favored in echolocating bats. Therefore, due to the ambiguity of the genes that cause echolocation in bats, more evidence remains to be discovered.
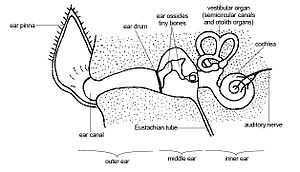
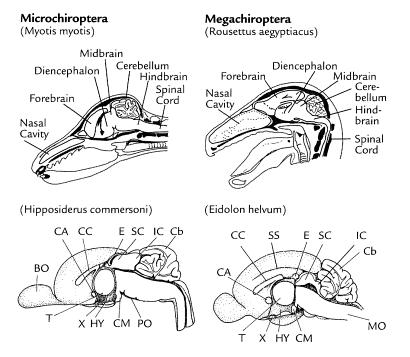
Anatomy
(1)[25], (2)[26], (3)[27], (4)[28], (5)[29], (6)[30]
Microbats use echolocation for catching prey. This requires a developed sense of hearing. Compared to that of a megabat, the pinnae of microbats are more complex and do not close to form a ring around the ear.[31] Microbats have a tragus inside their ear, which is usually absent in megabats.[32] Not only is the cochlea of echolocating bats larger, it also has an extra basal turn. [33] The extra basal turn is seen mostly in bats that produce constant frequency calls.[34]
Contrary to popular belief, echolocating bats are not blind. In order to avoid competition with birds, they hunt for prey at night. This lead to the increase in development for echolocation and decrease in development for vision. On the other hand, megabats use fruits and nectar for nutrition, so they don’t need to use echolocation. Megabats are thought to have lost their ability to echolocate as they invested more in the development of their sense of smell and sight. There is one genus of megabats called Rousettus that use tongue clicks to echolocate.(biology of bats) [35]
Low frequency sounds are mapped at the rear of the auditory cortex for microchiropterans. This explains why the frontal cortex in microbats is small and less developed than megabats. Megabats map sounds at the front of the auditory cortex. Megachiropterans have big eyes and well developed nocturnal vision, so the visual cortex is quite large. [36] Megabats have a generalized wing structure. The first 2 digits have claws and the metacarpal are similar in length to the phalanges.[37] The wing structure of microbats is specialized in different ways depending on the flight habits of the particular species.[38] The forelimbs of microbats move independently and allow them to be quick when on the ground. [39] An estimated 52 million year old fossil of an Eocene bat showed wings that were capable of basic flight. The cochlea of this fossil was very primitive and indicates that flight had developed long before echolocation.[40]
How it works
Echolocation is a sensory system in the form of biological sonar used by many species of bats and a wide range of other mammals. It is defined as the emission of sound pulses or ultrasonic calls. There are three ways that bats can vocalize, using different body parts to emit sound; nasal (5 families), lingual (one genus Rousettus) and laryngeal (12 families) [41].The return echo is subsequently processed via the auditory nervous system and gives acoustic images of the objects in the bats surroundings during foraging and navigation at night when vision is ineffective. It allows for detection, localization and even classification of prey. [42]
Among many bats species, variation in call design is related to ecological niche and reflects variation in habitat use. [43] In general, species that are foraging in open areas use calls that are long in duration, low in frequency and narrow in range. This combination favors the detection of large and distant prey. [44] The opposite is true of foraging in spatially complex habitats, with short, high frequency broadband signals used when close to obstacles that produce what is known as clutter and can result in forward masking .These are two very general call types that are suited for navigation and foraging but the actual mechanism of differences in calls are underlined by a number of acoustic features that can be varied according to conditions and species. [45].
Acoustic Features
These allow for not only identification of an object but also perception of target size, distance, horizontal direction and shape. Calls can also be altered according to behaviors such as emerging from roost vs foraging, size of flight area and presence of interfering signals. Acoustic features together make a call which is characteristic of a species.
Frequency
Pitch of sound measured in Hz or Khz. The range of frequencies exploited by bats is between 11kHz and 212kHz. A bat species dominant frequency is related to its prey. Lower frequencies are avoided in insectivorous bats because echoes from insect-sized targets are weak when the wavelength is longer than the insect wing length. There are two types of frequency structures, frequency modulated (FM) sweeps and constant frequency (CF) tones. They can be used individually or in combination and are effectively broadband or narrowband signals. [46]
Harmonic composition
A bat call can be composed of one or multiple frequencies, this is known as a harmonic series. Multiple frequency calls usually have a dominant frequency which is present at a higher intensity.
Duration and Pulse Interval
How long the pulse goes for and how much time passes between calls and is related to whether the bat is using high duty cycle or low duty cycle. Bats on low duty cycle (pulse emission <20% of the time) reduce call duration as they approach target to prevent overlap between outgoing signal and returning echo. [47] Pulse interval is also related to efficiency of Echolocation. The next call is not sent until the previous call has been received, which as the target approaches has a shorter time interval, this ends in the characteristic “terminal buzz” of approximately 200 pulses per second, put simply, call duration and interval decreases as target gets closer. Bats using high duty cycle (pulse emission <60% of the time, usually CF) take advantage of the doppler shift. Another method to prevent overlap is alternating between the call types which allows for an increase in call frequency without risk of return echo interference as bats have the ability to distinguish different calls. The range of call duration is wide, ranging from 0.2 to 100 milliseconds. [48]
Intensity
Call intensity in bats are among the highest recorded airborne vocalizations. Recordings of between 60 to 133dB have been recorded, the highest generally for species that fly fast in open. Species vary in call intensity, for example ‘whispering bats’ from the family Phyllostomidae have low intensity calls to avoid detection by their prey. A number of bats have the ability to adjust intensity mid-call. In addition the size of bat has also been found to have little effect on call intensity [49] [50]
Evolution of variation in calls
The evolution of different call types generated from the acoustic features listed above is extremely varied. Divergent and convergent evolution as well as adaptive radiation is observed. For example, high duty cycle is the most sophisticated form of biosonar (long CF tones terminated by broadband sweeps) and has evolved independently in horseshoe bats (Yinpterochiroptera) and mustached bats (Yangochiroptera), an example of convergence. This has also been seen in call design, bats which exploit similar ecological niches typically have similar call features such as in the insectivorous neotropical Myotis nigricans and the European Pipistrelle bats and their common foraging environment of edge-and-gap and open habitats. Differences in call frequency between closely related species has been suggested to be a result of divergent evolution in order to facilitate intra-specific communication and as a possible mechanism for sympatric speciation. The overall call diversity seen in echolocation calls between Yinpterochiroptera and Yangochiroptera is considered to illustrate adaptive radiation (28).
Evolutionary patterns of Echolocation
All though echolocation in bats appears to have evolved from a common ancestor, the appearance and development of this trait has shown a different pattern in other species present in the world of phylogeny. Echolocation in unrelated species alongside that of bats can be considered one of the most dramatic examples yet of ‘convergent evolution’. These unrelated lineages have independently evolved similar behaviours, traits or body parts in response to similar evolutionary pressures. [51] The species that exhibit echolocation include bats, dolphins, whales, and shrews; each with a specialized form that is custom suited to each individual in response to the challenges faced in the environments.
The most complex versions of the trait have evolved in species which must thrive in environments where vision can be ineffective (ex. night or dark, turbid water) using ultrasound for hunting, obstacle avoidance and orientation in space, but there are examples of simpler forms being used in shrews, rats, swiftlets and oilbirds. [52] These species use low amplitude sounds, which are shown to help investigate familiar habitat and possibly find food, if in close range. [53] Even though they are not related, the echolocation abilities of bats, dolphins and whales rely on the same changes to the same gene – Prestin. This gene plays a role in helping detect rebounding echoes, making outer hair cells shorter and stiffer than normal, allowing species to be exquisitely sensitive to the ultrasonic frequencies used in echolocation. [54] Changes to the Prestin gene have produced strikingly similar proteins which are shown to be common in all species mentioned above. Based on the DNA sequences of these Prestin versions, the phylogenetic tree would look like a normal mammal phylogenetic tree. However, when putting the common [protein discovery to use and converting the sequence to amino acids in these species, things look very different. Echolocating species would be placed very closely together, to the exclusion of other bats and whales that don’t use sonar.
It is very interesting to see such strong convergence at the genetic level, as the species are unrelated and they echolocate very differently. Bats create their sonar pulses using their voice box while whales pass air through their nasal bones. Bats send their calls through air and whales send their through water. However, regardless of these differences, they have somehow managed to accrue the same set of 14 amino acid changes, whether they have wings or flippers. These changes result in high-frequency selection and sensitivity which are strongly favoured in echolocating mammals, due to their uneffective vision in their environments. These similar changes in unrelated species show that there are only very few ways, if not only one, for Prestin to acquire the ability which allows these mammals to hear the ultrasonic sounds needed for biological sonar. [55]
Co-evolutionary examples
Despite all of these benefits that echolocation provides for the species that utilize it, selective pressure is causing prey to adapt to hear or deter theecholocation, helping them to avoid the predator. Some examples of these species are “eared” moths (ex. Tiger moths) who have evolved a noise making organ which produces high frequency pulses and clicks, interfering with bat echolocation. This noise startles the bat and mimics that of a noxious moth, signalling the bat to avoid it. [56] [57] Crickets also can evade echolocative predators through the auditory system located on the tibia of each foreleg, which allows them to hear bats at certain frequencies. [58] These adaptations are examples of a co-evolutionary arms race that could be taking place between the species of bats and their prey, and it could be possible in the future to see bats changing their frequencies in response to this detection.
References
- ↑ Speakman. "The evolution of flight and echolocation in bats: another leap in the dark." Mammal Review 31 (2001): 111-130. Web. 24 Nov. 2012. <http://www.abdn.ac.uk/ibes/speakman/pdf_docs/145.pdf>.
- ↑ "Eocene - Wikipedia, the free encyclopedia." Wikipedia, the free encyclopedia. N.p., n.d. Web. 24 Nov. 2012. <http://en.wikipedia.org/wiki/Eocene>.
- ↑ Speakman. "The evolution of flight and echolocation in bats: another leap in the dark." Mammal Review 31 (2001): 111-130. Web. 24 Nov. 2012. <http://www.abdn.ac.uk/ibes/speakman/pdf_docs/145.pdf>.
- ↑ "Bat - Wikipedia, the free encyclopedia."Wikipedia, the free encyclopedia. N.p., n.d. Web. 24 Nov. 2012. <http://en.wikipedia.org/wiki/Bat>
- ↑ Teeling. "Hear, hear: the convergent evolution of echolocation in bats?" TRENDS in Ecology and Evolution 24 (2009): 351-354. Science Direct. Web. 24 Nov. 2012.
- ↑ Teeling. "Hear, hear: the convergent evolution of echolocation in bats?" TRENDS in Ecology and Evolution 24 (2009): 351-354. Science Direct. Web. 24 Nov. 2012.
- ↑ "Evolution of high duty cycle echolocation in bats." The Journal of Experimental Biology . The Company of Biologists Ltd, n.d. Web. 24 Nov. 2012. <http://jeb.biologists.org/content/215/17/2935/F3.expansion>.
- ↑ "Evolution of high duty cycle echolocation in bats." The Journal of Experimental Biology . The Company of Biologists Ltd, n.d. Web. 24 Nov. 2012. <http://jeb.biologists.org/content/215/17/2935/F3.expansion>.
- ↑ Teeling. "Hear, hear: the convergent evolution of echolocation in bats?" TRENDS in Ecology and Evolution 24 (2009): 351-354. Science Direct. Web. 24 Nov. 2012.
- ↑ Teeling, and Jones. "The evolution of echolocation in bats." TRENDS in Ecology and Evolution 21 (2006): 149-156. Science Direct. Web. 24 Nov. 2012.
- ↑ Simmons, and Conway. "Chiroptera." The Tree of Life Web Project. Tree of Life Project, Jan. 2012. Web. 24 Nov. 2012. <http://tolweb.org/Chiroptera>.
- ↑ Teeling, and Jones. "The evolution of echolocation in bats." TRENDS in Ecology and Evolution 21 (2006): 149-156. Science Direct. Web. 24 Nov. 2012.
- ↑ Speakman. "The evolution of flight and echolocation in bats: another leap in the dark." Mammal Review 31 (2001): 111-130. Web. 24 Nov. 2012. <http://www.abdn.ac.uk/ibes/speakman/pdf_docs/145.pdf>.
- ↑ Teeling, Emma C.. "Hear, hear: the convergent evolution of echolocation in bats?." Trends in Ecology and Evolution 24.7 (2009): 351-354. ScienceDirect. Web. 24 Nov. 2012.
- ↑ Teeling, Emma C.. "Hear, hear: the convergent evolution of echolocation in bats?." Trends in Ecology and Evolution 24.7 (2009): 351-354. ScienceDirect. Web. 24 Nov. 2012.
- ↑ Teeling, Emma C.. "Hear, hear: the convergent evolution of echolocation in bats?." Trends in Ecology and Evolution 24.7 (2009): 351-354. ScienceDirect. Web. 24 Nov. 2012.
- ↑ Yong, Ed. "Echolocation in bats and whales based on same changes to same gene – Not Exactly Rocket Science." ScienceBlogs - Where the world turns to talk about science.. N.p., 25 Jan. 2010. Web. 24 Nov. 2012. <http://scienceblogs.com/notrocketscience/2010/01/25/echolocation-in-bats-and-whales-based-on-same-changes-to-sam/>.
- ↑ Coghlan, Andy. "Bats and dolphins separately evolved same sonar gene - life - 25 January 2010 - New Scientist." Science news and science jobs from New Scientist - New Scientist. N.p., 25 Jan. 2010. Web. 24 Nov. 2012. <http://www.newscientist.com/article/dn18428-bats-and-dolphins-separately-evolved-same-sonar-gene.html>.
- ↑ Jones, Gareth. "Molecular Evolution: Gene Convergence in Echolocating Mammals." Current Biology 20.2 (2009): R62-R64. ScienceDirect. Web. 24 Nov. 2012.
- ↑ Teeling, Emma C.. "Hear, hear: the convergent evolution of echolocation in bats?." Trends in Ecology and Evolution 24.7 (2009): 351-354. ScienceDirect. Web. 24 Nov. 2012.
- ↑ Jones, Gareth. "Molecular Evolution: Gene Convergence in Echolocating Mammals." Current Biology 20.2 (2009): R62-R64. ScienceDirect. Web. 24 Nov. 2012.
- ↑ Jones, Gareth. "Molecular Evolution: Gene Convergence in Echolocating Mammals." Current Biology 20.2 (2009): R62-R64. ScienceDirect. Web. 24 Nov. 2012.
- ↑ Teeling, Emma C.. "Hear, hear: the convergent evolution of echolocation in bats?." Trends in Ecology and Evolution 24.7 (2009): 351-354. ScienceDirect. Web. 24 Nov. 2012.
- ↑ Jones, Gareth. "Molecular Evolution: Gene Convergence in Echolocating Mammals." Current Biology 20.2 (2009): R62-R64. ScienceDirect. Web. 24 Nov. 2012.
- ↑ "Bat - Wikipedia, the free encyclopedia."Wikipedia, the free encyclopedia. N.p., n.d. Web. 28 Nov. 2012. <http://en.wikipedia.org/wiki/Bats>.
- ↑ Pettigrew, J. D., F. R. S., B. G. M. Jamieson, S. K. Robson, L. S. Hall, K. I. McNally, and H. M Cooper. "Phylogenetic relations between microbats, megabats and primates(Mammalia: Chiroptera and Primates)." Philosophical Transactions of the Royal Society of London 325.1229 (1989): 489-559. Print.
- ↑ Chittajallu, S.K., K. G. Kohrt, M. J. Palakal, and D. Wong. "Computational Model of the Bat Auditory Periphery."Mathl. Comput. Modelling 24.1 (1996): 67-68. Print.
- ↑ Altringham, John D.. "Evolution and diversity." Bats: from evolution to conservation. 2nd ed. New York: Oxford University Press, 2011. 9-13. Print.
- ↑ Jones, Gareth, and Emma C. Teeling. "The evolution of echolocation in bats."Trends in Ecology and Evolution 21.3 (2006): 149-155. Print.
- ↑ Pye, J. D.. "Hearing in Bats." Hearing mechanisms in vertebrates; a Ciba Foundation symposium.. Boston: Little, Brown, 1968. 70-77. Print.
- ↑ "Bat - Wikipedia, the free encyclopedia."Wikipedia, the free encyclopedia. N.p., n.d. Web. 28 Nov. 2012. <http://en.wikipedia.org/wiki/Bats>.
- ↑ Neuweiler, Gerhard. "5 | Central Nervous System." The biology of bats. New York: Oxford University Press, 2000. 128-133. Print.
- ↑ Altringham, John D.. "Evolution and diversity." Bats: from evolution to conservation. 2nd ed. New York: Oxford University Press, 2011. 9-13. Print.
- ↑ Pye, J. D.. "Hearing in Bats." Hearing mechanisms in vertebrates; a Ciba Foundation symposium.. Boston: Little, Brown, 1968. 70-77. Print.
- ↑ "Bat - Wikipedia, the free encyclopedia."Wikipedia, the free encyclopedia. N.p., n.d. Web. 28 Nov. 2012. <http://en.wikipedia.org/wiki/Bats>.
- ↑ Neuweiler, Gerhard. "5 | Central Nervous System." The biology of bats. New York: Oxford University Press, 2000. 128-133. Print.
- ↑ Altringham, John D.. "Evolution and diversity." Bats: from evolution to conservation. 2nd ed. New York: Oxford University Press, 2011. 9-13. Print.
- ↑ Lawlor, T.E.. "Order Chiroptera." http://www.eebweb.arizona.edu/Courses/Ecol485_585/Readings/Lawlor/Order_Chiroptera_T.E.Lawlor.pdf. N.p., n.d. Web. 24 Nov. 2012. <www.eebweb.arizona.edu/Courses/Ecol485
- ↑ Altringham, John D.. "Evolution and diversity." Bats: from evolution to conservation. 2nd ed. New York: Oxford University Press, 2011. 9-13. Print.
- ↑ "Bat - Wikipedia, the free encyclopedia."Wikipedia, the free encyclopedia. N.p., n.d. Web. 28 Nov. 2012. <http://en.wikipedia.org/wiki/Bats>.
- ↑ Yovel, Y., Geva-sagiv, M., & Ulanovsky, N. (2011). Click-based echolocation in bats: Not so primitive after all. Journal of Comparative Physiology, 197(5), 515-30. doi: http://dx.doi.org/10.1007/s00359-011-0639-4
- ↑ Gareth Jones, Echolocation, Current Biology, Volume 15, Issue 13, 12 July 2005, Pages R484-R488, ISSN 0960-9822, 10.1016/j.cub.2005.06.051. (http://www.sciencedirect.com/science/article/pii/S096098220500686X).
- ↑ Jones, Gareth , and Marc Holderied. "Bat echolocation calls: adaptation and convergent evolution." National Center for Biotechnology Information. N.p., n.d. Web. 24 Nov. 2012. <http://www.ncbi.nlm.nih.gov/pmc/article
- ↑ Gillam, Erin H., et al. "Bats Aloft: Variability in Echolocation Call Structure at High Altitudes." Behavioral Ecology and Sociobiology 64.1 (2009): 69-79. ABI/INFORM Dateline; ABI/INFORM Global; ABI/INFORM Trade & Industry; ProQuest Central; ProQuest European Business; ProQuest Natural Science Collection. Web. 24 Nov. 2012.
- ↑ Matthew A. Wund, Learning and the development of habitat-specific bat echolocation, Animal Behaviour, Volume 70, Issue 2, 1 August 2005, Pages 441-450, ISSN 0003-3472, 10.1016/j.anbehav.2004.11.009. (http://www.sciencedirect.com/science/article/pii/S0003347205001399)
- ↑ Simmons, James, and Roger Stein. "Acoustic imaging in bat sonar: Echolocation signals and the evolution of echolocation - Springer." Home - Springer. N.p., n.d. Web. 24 Nov. 2012. <http://link.springer.com/article/10.1007%
- ↑ Jones, Gareth , and Marc Holderied. "Bat echolocation calls: adaptation and convergent evolution." National Center for Biotechnology Information. N.p., n.d. Web. 24 Nov. 2012. <http://www.ncbi.nlm.nih.gov/pmc/article
- ↑ Simmons, James, and Roger Stein. "Acoustic imaging in bat sonar: Echolocation signals and the evolution of echolocation - Springer." Home - Springer. N.p., n.d. Web. 24 Nov. 2012. <http://link.springer.com/article/10.1007%
- ↑ Jones, Gareth , and Marc Holderied. "Bat echolocation calls: adaptation and convergent evolution." National Center for Biotechnology Information. N.p., n.d. Web. 24 Nov. 2012. <http://www.ncbi.nlm.nih.gov/pmc/article
- ↑ Surlykke, Annemarie, and Elisabeth Kalko. "Echolocating Bats Cry Out Loud to Detect Their Prey." National Center for Biotechnology Information. N.p., n.d. Web. 24 Nov. 2012. <http://www.ncbi.nlm.nih.gov/pmc/article
- ↑ Yong, Ed. "Echolocation in bats and whales based on same changes to same gene – Not Exactly Rocket Science." ScienceBlogs - Where the world turns to talk about science.. N.p., n.d. Web. 22 Nov. 2012. (http://scienceblogs.com/notrocketscience/2010/01/25/echolocation-in-bats-and-whales-based-on-same-changes-to-sam)
- ↑ Gareth Jones, Echolocation, Current Biology, Volume 15, Issue 13, 12 July 2005, Pages R484-R488, ISSN 0960-9822, 10.1016/j.cub.2005.06.051. (http://www.sciencedirect.com/science/article/pii/S096098220500686X).
- ↑ Gould, E., Negus, N. C. and Novick, A. (1964), Evidence for echolocation in shrews. J. Exp. Zool., 156: 19–37. doi: 10.1002/jez.1401560103 (http://onlinelibrary.wiley.com/doi/10.1002/jez.1401560103/abstract)
- ↑ Yong, Ed. "Echolocation in bats and whales based on same changes to same gene – Not Exactly Rocket Science." ScienceBlogs - Where the world turns to talk about science.. N.p., n.d. Web. 22 Nov. 2012. (http://scienceblogs.com/notrocketscience/2010/01/25/echolocation-in-bats-and-whales-based-on-same-changes-to-sam)
- ↑ Cell Press. "In bats and whales, convergence in echolocation ability runs deep." ScienceDaily, 27 Jan. 2010. Web. 22 Nov. 2012. ( http://www.sciencedaily.com/releases/2010/01/100125123219.htm)
- ↑ "Echolocation." University of Arizona . N.p., n.d. Web. 18 Nov. 2012. <www.eebweb.arizona.edu/Courses/Ecol485_585/Campbell_Echolocation%20lectures_2012.pdf>.
- ↑ Christine, Dell'Amore. ""Whispering" Bat Evolved to Trick Prey." Daily Nature and Science News and Headlines | National Geographic News. N.p., 1 Sept. 2010. Web. 22 Nov. 2012. <http://news.nationalgeographic.com/news/2010/08/100831-bats-whisper-moths-environment-animals-science/>.
- ↑ Lee A., Miller, and Surlykke, Annemarie. "How Some Insects Detect and Avoid Being Eaten by Bats: Tactics and Countertactics of Prey and Predator." University of Maryland. Version Vol. 51 No. 7. BioScience, n.d. Web. 17 Nov. 2012. <www.life.umd.edu/faculty/wilkinson/honr278c/PDF/Miller01.pdf>