Course:KIN 500
| Special Topics In Kinesiology | |
|---|---|
| HKIN 500 | |
| Section: | |
| Instructor: | Darren Warburton |
| Email: | darren.warburton@ubc.ca |
| Office: | |
| Office Hours: | |
| Class Schedule: | |
| Classroom: | |
| Important Course Pages | |
| Syllabus | |
| Lecture Notes | |
| Assignments | |
| Course Discussion | |
Cardiovascular Assessments During Rest and Exercise are novel tools for identifying health risk factors such as heart disease, cardiac abnormalities during rest or exercise, athletic performance and overall health and well being [1] . Cardiac stress test's such as Stress Echocardiography (stress echo) have become a standard practice for health care practitioners as it is an excellent identification tool for individuals with potential cardiovascular risk factors [2] . Cardiac assessments can be beneficial not only for the clinical population but also as preventive measures in undiagnosed individuals with a family history of heart disease. Athletes can also benefit from certain cardiac measurements. Assessment of possible sudden cardiac abnormalities during competition, over training syndrome and overall central nervous system performance can be examined using tools such as an Electrocardiogram (EKG) and Heart Rate Variability (HRV) [3] .
12-Lead EKG
An electrocardiogram EKG or ECG is a non-invasive test that measures the electrical activity of the heart.[4] Cardiac monitors depict the heart's electrical impulses as patterns of waves.[5] The electrical impulses present on the skin have a very low voltage and will need to be amplified by the EKG machine. The EKG machine will record the electrical activity and print what is called a rhythm strip or an EKG strip. The polarization and depolarization of the cardiac cells is translated onto the EKG strip as a waveform. This waveform can then be used to determine the rate of heart beats, the regularity of the heart beats, the size of the chambers, and any damage or abnormalities in the heart.[5] [6] The 12-Lead EKG views the heart in two planes, the frontal plane and the horizontal plane.The vector leads look at the horizontal plane while the limb leads look at the frontal plane. [7]
Indications
Some indications to take a resting EKG are as follows:
- Symptoms of myocardial infarction or a pulmonary embolism[8]
- Symptoms that suggest heart problems such as chest pain, dsypnea, shortness of breath, syncope/ near syncope [9]
- Unusual heart sounds or murmurs found by a doctor [9][8]
- Known or suspected cardiac abnormalities
- An EKG may also be a part of a routine check-up or pre- and post-op screening[9]
Risks
A resting EKG is a quick, non-invasive method to measuring heart function and has little to no risks involved with testing. [10] No electricity is sent through the body, so there is no risk of shock. [10] [9] The subject may develop a rash or irritation where the electrodes were placed.[10][9] As with any test, there may be risks due to other medical conditions. Be sure to consult with a doctor before testing if there are any concerns about the procedure.
Subject Preparation
A resting EKG will have little preparation beyond skin preparation for electrodes.[9] The technician should give a detailed explanation of the procedure and answer any questions the subject may have. A medical history should be taken to determine possible effects of any cardioactive drugs and past conditions.
Before completing a stress test, the subject should be instructed not to eat or smoke at least three hours before the test, however, he may drink water as needed. The subject should also be told to dress for exercise, especially with proper footwear. The subject should participate in no unusual physical exertion at lease 12 hours before testing. For diagnostic purposes the subject may be told to stop taking some medications by his physician, due to the effects of some drugs to attenuate the exercise response. However, most subjects undergoing an exercise tolerance test will be taking their usual medications.[6] Be sure that the subject followed his physicians orders to continue or stop taking medications. A history should be taken on each subject to determine possible effects of any cardioactive drugs and past conditions. A brief history should be taken on each subject to rule out any contraindications to testing, and to ensure the correct physician supervision is there for the testing. Special considerations should be made for patients with elevated blood pressure.
A detailed explanation of the testing procedure should be given to the patient in order to outline the risks and possible complications of testing. The subject should also fully understand the protocols for the test he is about to complete.
Only well trained staff should conduct a stress test and the technicians should be familiar with normal and abnormal responses during the test. Individuals that are trained in advanced cardiopulmonary resuscitation (CPR) should be readily available throughout the duration of the test. As with any procedure that poses a risk to the subject, the technicians should be certain that the subject fully understands both the risks and benefits. Written consent should be obtained prior to the start of testing.
Lead Placement
Skin Preparation
The most crucial point of the EKG machine's recording system is the interface between the electrode and the skin. By removing the most superficial layer of the skin, the resistance can be significantly decreased and, therefore, the noise ratio also is decreased. If needed, the areas of electrode placement should be shaved and then sterilized. After the skin has dried, the exact locations for the electrodes should be marked, and the superficial layer of the skin removed by a dry paper towel or other rough abrasive material designed specifically for use on the skin.
Electrodes and Cables
Electrodes are adhesive pads that are designed to attach to the subject's skin, and contain a conductive gel. These electrodes are then connected to the EKG machine by cables, called leads. The leads tend to be colour-coded to be more user friendly. One should note that most leads have a life span of about one year, and should be replaced often. When checking equipment prior to testing, it is important to note that the electrode gel is moist.
Leads
| Electrode Name | Placement |
|---|---|
| Right Arm | Between the right shoulder and wrist, avoiding bony prominences |
| Left Arm | Between the left shoulder and wrist, avoiding bony prominences (bone is a poor conductor) |
| Right Leg | Between the right hip and ankle |
| Left Leg | Between the left hip and ankle |
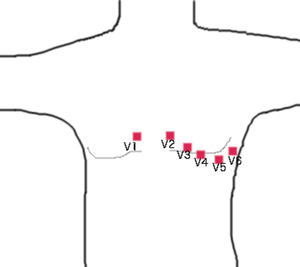
| V1 | Forth intercostal space, just to the right of the sternum |
| V2 | Forth intercostal space, just to the left of the sternum |
| V3 | Between V2 and V4 |
| V4 | Fifth intercostal space, mid-clavicular line |
| V5 | Anterior axillary line, level with V4 |
| V6 | Mid-axillary line, level with V4 and V5 |
Interpretation
EKG Graph Paper
The EKG graph paper is standardized to all machines, and leaves the machine are a rate of 25mm per second. Each small square is 1 mm high and wide, and is 0.04 seconds in length. Dark lines can be found every 5 small squares, creating larger squares outlined by these dark lines. The larger squares measure 5mm by 5mm and are 0.20 seconds in length. Both time and amplitude are measured and recorded on the EKG strip. You will find time along the horizontal line, and amplitude (or voltage) on the vertical line. Amplitude is measured in millivolts: two large squares = 1 mV; 1 mV = 10mm.
EKG Waveforms
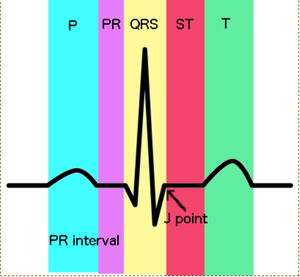
A waveform refers to any movement away from the baseline that is recorded on the EKG strip. The baseline is the straight line on the EKG strip, and represents the beginning and end point of all waves and is referred to as isoelectric. A positive deflection is above the baseline and a negative deflection is below the baseline. [5] A depolarizing electrical impulse that is travelling towards a positive electrode will create a positive deflection. Conversely, a depolarizing electrical impulse travelling away from a positive electrode will create a negative deflection on the EKG strip. [11]
| Wave | Description | Normal Duration |
|---|---|---|
| P Wave | The SA node of the heart fires first during a normal cardiac cycle. The firing sends the electrical impulse outward to stimulate both atria and manifests as the P Wave. On the EKG strip, the P Wave is a smooth, rounded, positive deflection. The P Wave represents the depolarization of both the left and right atria. [5] | <0.11 seconds [11] [5] |
| PR Interval PRI | The PRI represents the time interval necessary for the impulse to travel from the SA node, through the inter-nodal pathways in the atria and downward to the ventricles. The PRI is said to be representative of the distance from the beginning of the P wave to the beginning of the QRS complex. | 0.12-0.20 seconds (3-5 small squares) [11] [5] |
| QRS Complex | The QRS complex consists of the Q, R, and S waves, and represents the conduction of the electrical impulse from the Bundle of His throughout the ventricle muscles, ventricular depolarization. It is measured from the beginning of the Q wave to where the S wave meets the baseline.[5] The Q wave is seen as the first downward deflection following the PRI. The R wave is the first upward deflection of the QRS complex and is normally the largest defection seen. The S wave is a downward deflection that immediately follows the R wave. | ~0.12 seconds (~ 3 small squares)[5] |
| J Point | The point at which the QRS complex meets the ST segment is known as the J point, and is a valuable landmark in 12-lead EKG interpretation. When evaluating the ST segment for elevation or depression, it begins with analysis of the J point.[5] | N/A |
| ST Segment | The time interval during which the ventricles are depolarized and ventricular repolarization begins is known as the ST segment. Normally the ST segment is isoelectric, consistent with the baseline. Elevation of the ST segment is a major EKG change seen in an acute myocardial infarction.[5] | 0.08 -0.12 seconds [5] |
| T Wave | The T wave follows the ST segment and is representative of ventricular repolarization. The T wave is normally seen as a slightly asymmercial, rounded, positive deflection. Ventricular repolarization is an electrical event with no associated activity of the ventricular musculature, therefore, the T wave is also known as the resting phase.[5] | 0.10-0.25 seconds [11] |
Possible Outcomes
Heart Rate Variability (HRV)
Heart Rate Variability (HRV) has demonstrated to be a non-invasive technique that can assess the autonomic nervous system. HRV identifies and represents the variations between consecutive heart beats [12]. These variations in R-R intervals have been thought to express a measure of balance between the parasympathetic and sympathetic nervous system [13]. Every R-R interval collected through the use of and 12 lead Electrocardiography or heart rate monitoring system can be analyzed using frequency, geometric, and time domain analysis in an attempt to identify autonomic fatigue [14].
R-R Intervals
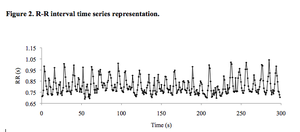
The first step in the analysis process is the measurement of each R-R time interval; these intervals are measured in milliseconds (ms) (Figure 1). Each R-R time interval represents a cardiac beat or a time measurement through the cardiac cycle collected (Figure 2). It should be stressed that whatever R-R series is chosen for analysis it should be edited prior to uploading the raw data into any HRV software [15]. It has been demonstrated that a single ectopic or missed beat during a 5 minute recording can skew HRV variables up to 50% [16]. Choosing a 10 second R-R interval strip with minimal fluctuations can help promote true HRV indices.
Methods for HRV analysis
Time Domain
This method of analysis involves time domain variables derived from the differences between R-R intervals or directly from the measurement of the R-R interval [17] .
Calculated from the differences in R-R intervals [18] [19]
NN50
- The amount of adjacent R-R intervals that differed by more than 50ms
pNN50 (%)
- The percentage of adjacent R-R intervals
- There is a solid correlation with High Frequency (HF) power.
rMSSD (ms)
- A root mean square in successive difference. The square root of the mean of the sum of the difference between adjacent R-R intervals in a complete recording [20]
- Assesses change in short term cycles. There is a strong correlation with HF power. [21]
Calculated from the measurement of R-R intervals [22]
Mean RR (ms) (s)
- The mean R-R intervals between all normal beats that have been selected in an interval series.
Mean Heart Rate (1/min)
- The mean heart rate of a selected series
RRmax – Rrmin (ms) (s)
- The difference between the average minimum R-R interval and the average maximum R-R interval.
SDNN (ms) (s)
- The standard deviation (SD) of all the normal R-R intervals from a selected series.
- Can represent all the cyclic elements responsible for the variability during the recording time frame.
SDNN index (ms) (s)
- The mean of the SD of all normal R-R intervals during 5 minutes segments of the complete recording period
SDANN (ms) (s)
- The SD of the average normal sinus R-R intervals during 5 minutes segments of the complete recording period.
- Can project changes in cycles longer than 5 minutes
Geometric Method
Geometric methods examine the R-R intervals in certain geometric patterns. These patterns can consist of density distribution of R-R interval duration or sample density distribution of difference between R-R intervals adjacent to each other (Figure 3). One of the benefits of using geometric analysis is its ability to reduce the effect of periodically incorrect R-R intervals through focusing on the major peak of the sample density curve [23] . With this advantage comes the disadvantage of requiring a large number of R-R intervals to assess [24]. The current method for geometric analysis may be unreasonable when assessing transient changes in HRV as a minimum of 24 minutes is required with 24 hours being desired .
Line of identity
- A 45 degree line to the normal axis
SD1
- The Standard deviation of all the points that are perpendicular to the line of identity.
- This can identify short term R-R interval variability caused by breathing during HRV, [25].
SD2
- The standard deviation along the line of identity.
- This can identify any long term variability
Frequency Domain
Frequency domain analysis can identify the effects of the sympathetic and parasympathetic systems ability to effect HRV [26]. It is thought this method of HRV analysis can elicit greater information on both the sympathetic and parasympathetic autonomic nervous system [27]. The frequency domain, labelled the power spectral density (PSD) identifies the recurring oscillations of heart rate signals at different amplitudes and frequencies. This information can demonstrate the power of the heart’ normal sinus rhythm [28] . Identifying the power involves breaking down the chosen series of R-R intervals into sums of sinusoidal functions involving different amplitudes and frequencies. Power spectral analysis can be completed through two different methods
- 1 Parametric Method: This method involves and auto regression model resulting in a continuous smooth spectrum of activity [29]
- 2 Nonparametric Method: This method involves a fast Fourier transformation that is marked by distinct peaks for the several frequency components. This allows any complex wave to be dissected into sine waves that when combined together provide the original complex wave.
A power spectrum encompasses frequency bands ranging from 0 – 0.5Hz that can be dissected into four frequency bands such as ultra-low frequency (ULF), very low frequency (VLF), low frequency (LF) and high frequency (HF). When assessing 5-10 minutes of HRV, deemed short term analysis, these measurements are characterized by HF,LF and VLF. Assessments lasting longer than 10 minutes involve ULF.
Ultra Low Frequency (ULF)
- A frequency band < 0.003 Hz
- Can identify very low oscillations in frequency which are controlled through circadian rhythm.
Very Low Frequency (VLF)
- A frequency band that falls between 0 – 0.04 Hz
- Can identify certain fluctuations that occur slowly and that are controlled through the circadian rhythms and possible peripheral vasomotor and thermoregulations factors.
Low Frequency (LF)
- A frequency band that falls between 0.04 – 0.15
- Can identify parasympathetic and sympathetic activity
High Frequency (HF)
- A frequency that falls between 0.15 – 0.4
- Can identify parasympathetic activity and vagal influence on heart rate
Peak Frequency (Hz)
- Represents the peak frequencies for VLF, LF and HF.
Power (ms2)
- Represents the power values in ms2 and in percentile units for VLF, LF and HF.
- The components of the spectrum are calculated in terms of frequency and amplitude which are assessed by the power amplitude of each component.
LF/HF
- Represents the ratio of LF and HF power in ms2.
- Can identify sympathovagal control over HR and tone of the Sinoatrial Node (SA).
- Can identify sympathetic dominance when values are high.
- Can identify vagal dominance when values are low.
Total Power (ms2)
- Represents the addition of HF, LF and VLF power values.
- Can identify R-R interval variance
Normalized Power Units (nu)
- Both HF and LF powers can be represented in normalized units (nu)
- nu can help suppress any noise caused by artifacts minimizing potential changes in TP and HF & LF powers.
HFnu= 100x HF/(TP-VLF)
LFnu=100xLF/(TP-VLF)
| Frequency Band | Peak (Hz) | Power (ms2) | Power (%) | Power (nu) |
|---|---|---|---|---|
| VLF | 0.0 | 25 | E8.2 | |
| LF | 0.0918 | 138 | 45.8 | 46.6 |
| HF | 0.1992 | 139 | 46.0 | 46.9 |
| LF/HF | 0.994 | |||
| Total Power | 302 |
Use & Considerations
- A heart rate monitor or monitoring system is needed to collect and export resting R-R interval data.
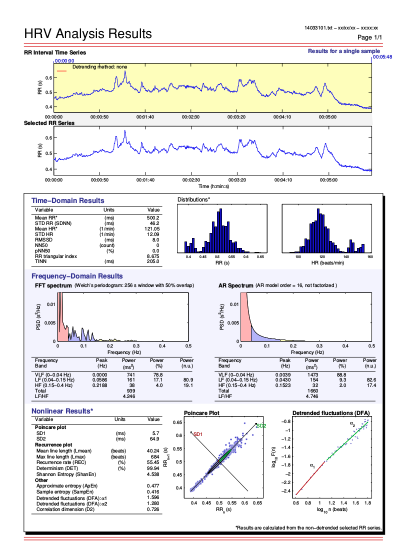
- Software such as Kubios HRV - Heart Rate Variability Analysis Software is required to import and analyze R-R interval data (Figure 4). http://kubios.uef.fi/
- When collecting data one should standardized the time of collection. This time frame should be chosen based on the intent behind analysis as body hydration and environmental stress are some factors that will fluctuate throughout the day altering HRV [30].
- 10 minutes of laying supine in a quiet comfortable environment is required for R-R interval collection.
- Breathing rate and depth should be standardized and relaxed. When collection is immediate upon waking up it is important not to fall back to sleep as this will alter the R-R interval time frames [31]..
- During collection it is best that the individual does not pay attention to their own HR monitor or screen.
- It is recommended during collection that all electronic sources are removed from the individual and moved away from the general vicinity in order to reduce potential EKG noise [32]
- It is important to establish baseline values to help identify positive and negative changes in HRV variables (ie. RMSSD, HF, LF, TP). This can be accomplish through collecting data during a stressful and relaxing week which can identify upper and lower values.
Stress Echocardiogram
Echocardiogram is an ultrasound of the heart at rest. It is ideally suited for the evaluation of cardiac mechanisms because of its dynamic nature [33]. It is a non-invasive, reliable test that provides a wealth of information examining the structure and function of the heart chambers, valves, and aorta. A Stress Echocardiogram is an echocardiogram examining what occurs when you place stress on the heart. This can be done with exercise or pharmacologically. Stress echo is suggested to be a more sensitive method for detecting and evaluating cardiac injury [34]. Today, it has developed into a robust and reliable technique because of its advances in image acquisition, digital display and the development of contrast imaging [35]. It is useful for identifying patients with known or suspected coronary artery disease, determining prognosis and examining the mechanisms that are causing decreased exercise performance [36]. Stress echo assesses overall exercise capacity, standard indices of cardiac function, indices of cardiac contractility, stroke volume, and cardiac output [37]. Information gained from stress echo should be an additive to other clinical variables, exercise duration, EKG changes and resting ventricular function [36].
Role of Stress Echocardiogram
Coronary Artery Disease
The most common use of the stress echo is diagnosing coronary artery disease (CAD) and risk stratifying those with suspected or established CAD [35]. Rest and stress images of global and regional left ventricular size, shape and function are examined to detect any abnormalities [36]. The hallmark of myocardial ischemia is reduced systolic wall thickness when myocardial demand exceeds myocardial blood supply [35]. Using Doppler methods, strain (myocardial deformation) and strain rate can be calculated and is used to define the transmural extent of myocardial infarction and the presence of viable myocardium [33]. The diagnosis of CAD is based on the detection of either a resting or inducible wall motion abnormality [36]. Normally, during stress the left ventricular size becomes smaller when compared to rest, while the shape is maintained and there is increased systolic wall thickening [35]. Conversely, in a pathological heart, a dilated ventricle, change in cavity shape and/or a notable reduction of systolic wall thickening is noticed [35]. If these abnormalities are displayed at rest it implies a sufficient infarction occurred or sufficient ischemia, indicating systolic function is abnormal or severe CAD [36]. If these changes are evoked at times of stress, this implies the presence of obstructive CAD [36].
Cardiac Risk Stratification
Subclinical Disease In subclinical disease, such as those with hypertension and diabetes, analysis of regional and global ventricular function at peak exercise is a powerful predictor of subsequent cardiac events [36]. An exercise ejection fraction of <50% indicates a significantly worse prognosis [36]. In cancer patients stress echocardiography can also be used to detect changes in cardiac function, contractility indices, and cardiac output to understand subclinical dysfunction [37].
Post-Acute Myocardial Infarction Stress echo can aid in the post-acute myocardial infarction prognosis by detecting the presence of residual or remote ischemia with stress induced wall motion abnormalities [38]. A worse prognosis is indicated by the extent of resting wall motion abnormalities, inducible ischemia and non-viability [39].
Pre-operative risk assessment The most important clinical predictors of cardiac death or myocardial infarction are previous infarction, angina, heart failure, and diabetes. The addition of a stress echo has been shown to improve risk stratification of patients prior to vascular or non-vascular surgery [40]. With stress testing, a low ischemic threshold and <70% of age predicted maximal heart rate is the strongest predictor of cardiac events; stress echo adds to the prognostic power [36]. Pharmacologic stress testing is usually preferred because the majority of these patients cannot adequately exercise.
Myocardial Viability
Dobutamine stress echo is commonly used to assess myocardial viability; dysfunctional myocardium with limited or absent scarring that has the potential for functional recovery [41]. Dobutamine increases myocardial oxygen demand. Dysfunctional segments that are viable can be demonstrated by increased contractility when a low dose dobutamine is injected. [41]. At higher doses of dobutamine, plus atropine to increase heart rate, wall motion may further improve or diminish. In the presence of flow-limiting stenosis there will be a demand/supply mismatch indicating inducible ischemia and subsequent deterioration of regional function [35]. A low likelihood of recovery is suggested when there is no change in contractility with either dose [36].
Non-Ischemic Disease
Hypertrophic Cardiomyopathy In asymptomatic or minimally symptomatic HCM patients, exercise stress testing, using Doppler techniques, provides excellent risk stratification [42].
Valvular Heart Disease The majority of patients with valvular stenosis have a conclusive evaluation based on resting echocardiogram and Doppler evaluation. Stress echo can be useful in providing additional information that occurs with exercise in situations of unexplained symptoms and moderate mitral stenosis at rest [43]. Pulmonary artery pressure can also be evaluated during exercise from the tricuspid regurgitation jet [36]. In severe aortic stenosis and depression of ventricular function, stress echo can provide re-evaluation of valvular hemodynamics and contractility to determine if the reduction in blood flow is exaggerating the reduction in the orifice area of the stenotic valve [44].
Methodology
Stress Echocardiography testing can be performed using a physiological (treadmill, semi-supine or upright cycle ergometry) or pharmacologic stress.
Treadmill stress echo, images are taken pre exercise and within 45 to 60 seconds post exercise. The disadvantages of this method are physiological responses during exercise are not assessed and the short time frame to take an image after completion of the test. If the image is not captured immediately, a wall motion abnormality may be missed if it resolves quickly (i.e. milder or single-vessel stenosis).
Semi-supine or upright cycle ergometry stress echo allows measures to be taken while the patient is exercising. This is usually completed 1.5 minutes into each stage of the exercise test protocol as well as pre and post exercise. The advantage with this method is a greater chance of detecting ischemia and sub clinical cardiac dysfunction [37].
Pharmacologic stress echo can be substituted when patients are incapable of exercise. The most commonly used agents are dobutamine and dipyridamole. Dobutamine has a primary impact on contractility myocardial oxygen [36]. Dipyridamole may be used as an alternative, but it produces infrequent wall thickening abnormalities [35]. Atropine is an agent that can be used with dobutamine and dipyridamole to increase the heart rate, mimicking exercise [36].
Techniques
Ultrasound waves transmitted into the body travel with a distinct velocity and are reflected at interfaces between tissues of different density (e.g. blood or myocardium) [45]. Two-dimensional and three-dimensional images of a cross-sectional area can be generated. These can be created in a parasternal long or short axis view or from an apical 2-chamber or 4-chamber view to examine the structure and function of the ventricles, valves, and aorta. Doppler imaging and tissue Doppler imaging (TDI) assesses the velocity of blood flow and myocardial tissue motion, respectively, using varying frequencies and amplitudes [46].
Two-Dimensional (2D) Echocardiography
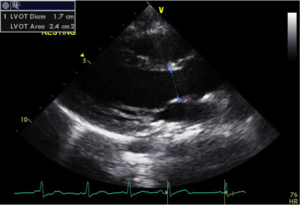
2D Echo displays a cross sectional area using horizontal and vertical dimensions. Real-time motion of the heart’s structures can be observed, measured and evaluated (Figure 1).
Three-Dimensional (3D) Echocardiography
3D Echo uses horizontal, vertical and depth dimensions. It allows rotation and visualization from multiple perspectives, allowing a more accurate assessment of left ventricle shape and function. 3D echo is vulnerable to suboptimal image quality during stress testing due to higher heart rates and therefore is less commonly used [47].
M-Mode echocardiography
M-Mode Echo is the simplest form and produces an image similar to a tracing than an actual picture [48]. It can be performed in the parasternal short and long axis view to measure the dimensions of ventricles, size of the heart and the thickness of heart walls (Figure 2).
Doppler Imaging
Pulsed Doppler imaging assesses velocity of blood flow [49] (Figure 3). Blood flow velocities can be used to calculate stroke volume and detect valvular regurgitation [49]. Color Doppler is often used to optimally visualize regurgitation, with the color denoting the direction and character of the flow [48]. Tissue Doppler Imaging (TDI) permits an assessment of myocardial velocities. Tissue velocity measurements can identify strain or strain rate to identify areas of reduced heart muscle contractility [46] .
Analysis
Analysis of stress echo has several levels of complexity. An experienced sonographer is required to obtain optimal measurements and images. The measurements and tracings acquired by the sonographer are analyzed by the echo machine software. Ventricluar measurements and function is measured with a 2D echo and M-mode. Valvular function is examined with 2D echo and Doppler imaging.
- Qualitative assessment is used to diagnose CAD. Different views of the heart can describe regional wall motion as:
- Normal, hypokinetic, akinetic, or dyskinetic.
- Stroke volume (SV) = EDV - ESV
- SV is determined by Doppler signal using the velocity-time integral and aortic cross-sectional area (π x aortic diameter2/4) [49].
- This method is based on the assumption that the blood flow determined from the Doppler is distributed throughout the cross section of the vessel so that the product of the area under the velocity curve times the cross-sectional area of the vessel is equal to the volume of blood passing through the vessel [49].
- Left ventricular ejection fraction (EF)
- Ejection fraction (EF) = [(end diastolic volume (EDV) – end- systolic volume (ESV) / end-diastolic volume] x100%
- Cardiac output
- Q - SV x HR
- Heart Rate: determined using R-R interval of the ECG.
References
- ↑ Allen, W., Aronow,W., Goodman,P., Stinson, P. 1980. Five year follow up of maximal treadmill stress test in asymptomatic men and women. Circulation: Journal of the American Heart Association. 62:522-527.
- ↑ Tsang,T., Barnes,M., Gersh,B., Takemoto,Y., Rosales,G., Bailey, K., Seward, J. 2003. Prediction of Risk for First Age Related Cardiovascular Events in an Elderly Population: The Incremental Value of Echocardiography. Journal of the American College of Cardiology. 42:7
- ↑ Pichot, V., Roche, F., Gaspoz, JM., Enjolras, F., Antoniadis, A., Minini, P., Costes, F., Busso, T., Lacour, JR., Barthelemy, JC. 2000. Relation between heart rate variability and training load in middle distance runners. Medicine & Science in Sports & Exercise.
- ↑ [1], American Heart Association.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 [2], Understanding 12-Lead EKGs, a Practical Approach. Beasley and West, Brady Books, 2001.
- ↑ 6.0 6.1 Society of Cardiovascular Science & Technology , Recommendations For Clinical Exercise Tolerance Testing, 2011.
- ↑ [3],Canadian Cardiac Society .
- ↑ 8.0 8.1 , Rodger M, Makropoulos D, Turek M, et al. (October 2000). "Diagnostic value of the electrocardiogram in suspected pulmonary embolism". Am. J. Cardiol. 86 (7): 807–9, A10.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 [4], The National Heart, Blood, and Lung Institute.
- ↑ 10.0 10.1 10.2 [5], Medline Plus, A service of the U.S. National Library of Medicine and National Institutes of Health.
- ↑ 11.0 11.1 11.2 11.3 [6] , The six second ECG Workbook Nursecom Educational Technologies and SkillStat Learning Inc. 2004.
- ↑ Reed, M.J., Robertson, C.E. and Addison, P.S. 2005. Heart rate variability measurements and the prediction of ventricular arrhythmias. Q.J. Med, 98: 87-95.
- ↑ Reed, M.J., Robertson, C.E. and Addison, P.S. 2005. Heart rate variability measurements and the prediction of ventricular arrhythmias. Q.J. Med, 98: 87-95.
- ↑ Reed, M.J., Robertson, C.E. and Addison, P.S. 2005. Heart rate variability measurements and the prediction of ventricular arrhythmias. Q.J. Med, 98: 87-95.
- ↑ Task Force of the European society of cardiology and the North American society of pacing and electrophysiology. 1996. Heart rate variability –standards of measurement, physiological interpretation, and clinical use. Circulation, 93(5), 1043-1065.
- ↑ Buchheit, M., Voss, S. C., Nybo, L., Mohr, M., and Racinais, S. 2011. Physiological and performance adaptations to an in-season soccer camp in the heat: associations with heart rate and heart rate variability. Scand. Journal Medicine Science and Sports 21, e477–e485. doi: 10.1111/j.1600-0838.2011.01378.x
- ↑ Lopes, P. and White, J. 2006. Heart rate variability: measurement methods and practical implications. In Physiological Assessment of Human Fitness (2nd edition). Edited by P.J. Maud and C. Foster. Human Kinetics, Champaign, IL.
- ↑ Lopes, P. and White, J. 2006. Heart rate variability: measurement methods and practical implications. In Physiological Assessment of Human Fitness (2nd edition). Edited by P.J. Maud and C. Foster. Human Kinetics, Champaign, IL.
- ↑ Kleiger, R.E., Stein, P.K., Bosner, M.S. and Rottman, J.N. 1995. Time-Domain Measurements of Heart Rate Variability. In Heart Rate Variability. Edited by M.M. Malik and A.J. Camm. Futura Publishing Company, Inc, Armork, New York. pp.33-45.
- ↑ Lopes, P. and White, J. 2006. Heart rate variability: measurement methods and practical implications. In Physiological Assessment of Human Fitness (2nd edition). Edited by P.J. Maud and C. Foster. Human Kinetics, Champaign, IL.
- ↑ Lopes, P. and White, J. 2006. Heart rate variability: measurement methods and practical implications. In Physiological Assessment of Human Fitness (2nd edition). Edited by P.J. Maud and C. Foster. Human Kinetics, Champaign, IL.
- ↑ Lopes, P. and White, J. 2006. Heart rate variability: measurement methods and practical implications. In Physiological Assessment of Human Fitness (2nd edition). Edited by P.J. Maud and C. Foster. Human Kinetics, Champaign, IL.
- ↑ Malik, M. 1995. Geometrical Methods for Heart Rate Variability Assessment. In Heart Rate Variability. Edited by M.M. Malik and A.J. Camm. Futura Publishing Company, Inc, Armork, New York. pp.47-61.
- ↑ Malik, M. 1995. Geometrical Methods for Heart Rate Variability Assessment. In Heart Rate Variability. Edited by M.M. Malik and A.J. Camm. Futura Publishing Company, Inc, Armork, New York. pp.47-61.
- ↑ Malik, M. 1995. Geometrical Methods for Heart Rate Variability Assessment. In Heart Rate Variability. Edited by M.M. Malik and A.J. Camm. Futura Publishing Company, Inc, Armork, New York. pp.47-61.
- ↑ Lopes, P. and White, J. 2006. Heart rate variability: measurement methods and practical implications. In Physiological Assessment of Human Fitness (2nd edition). Edited by P.J. Maud and C. Foster. Human Kinetics, Champaign, IL.
- ↑ Lopes, P. and White, J. 2006. Heart rate variability: measurement methods and practical implications. In Physiological Assessment of Human Fitness (2nd edition). Edited by P.J. Maud and C. Foster. Human Kinetics, Champaign, IL.
- ↑ Reed, M.J., Robertson, C.E. and Addison, P.S. 2005. Heart rate variability measurements and the prediction of ventricular arrhythmias. Q.J. Med, 98: 87-95.
- ↑ Niskanen, J.P., Tarvainen. M.P., Ranta-aho, P.O. and Karjalainen, P.A. 2004. Software for advanced HRV analysis. Compter Methods for Programs in Biomedicine, 76, 73-81.
- ↑ Lopes, P. and White, J. 2006. Heart rate variability: measurement methods and practical implications. In Physiological Assessment of Human Fitness (2nd edition). Edited by P.J. Maud and C. Foster. Human Kinetics, Champaign, IL.
- ↑ Lopes, P. and White, J. 2006. Heart rate variability: measurement methods and practical implications. In Physiological Assessment of Human Fitness (2nd edition). Edited by P.J. Maud and C. Foster. Human Kinetics, Champaign, IL.
- ↑ Lopes, P. and White, J. 2006. Heart rate variability: measurement methods and practical implications. In Physiological Assessment of Human Fitness (2nd edition). Edited by P.J. Maud and C. Foster. Human Kinetics, Champaign, IL.
- ↑ 33.0 33.1 Mor-Avi, V., et al., Current and Evolving Echocardiographic Techniques for the Quantitative Evaluation of Cardiac Mechanics: ASE/EAE Consensus Statement on Methodology and Indications. Journal of the American Society of Echocardiography, 2011. 24(3): p. 277-313.
- ↑ Sharkey, A.M., et al., Cardiac rehabilitation after cancer therapy in children and young adults. The American Journal of Cardiology, 1993. 71(16): p. 1488-1490.
- ↑ 35.0 35.1 35.2 35.3 35.4 35.5 35.6 ,Senior R., et al., Stress echocardiography for the diagnosis and risk stratification of patients with suspected or known coronary artery disease: a critical appraisal. Supported by the British Society of Echocardiography. Heart (British Cardiac Society), 2005. 91(4): p. 427-436.
- ↑ 36.00 36.01 36.02 36.03 36.04 36.05 36.06 36.07 36.08 36.09 36.10 36.11 36.12 , Armstrong W.F. and W.A. Zoghbi, Stress echocardiography: current methodology and clinical applications. Journal of the American College of Cardiology, 2005. 45(11): p. 1739
- ↑ 37.0 37.1 37.2 De Souza, A.M., et al., A stress echocardiography study of cardiac function during progressive exercise in pediatric oncology patients treated with anthracyclines. Pediatric blood & cancer, 2007. 49(1): p. 56-64.
- ↑ Sicari, R., et al., Exercise-electrocardiography and/or pharmacological stress echocardiography for non-invasive risk stratification early after uncomplicated myocardial infarction. A prospective international large scale multicentre study. European heart journal, 2002. 23(13): p. 1030-1037.
- ↑ Picano, E., et al., Prognostic value of myocardial viability in medically treated patients with global left ventricular dysfunction early after an acute uncomplicated myocardial infarction: a dobutamine stress echocardiographic study. Circulation, 1998. 98(11): p. 1078-1084.
- ↑ Das, M.K., et al., Assessment of cardiac risk before nonvascular surgery: dobutamine stress echocardiography in 530 patients. Journal of the American College of Cardiology, 2000. 35(6): p. 1647-1653.
- ↑ 41.0 41.1 Camici, P.G., S.K. Prasad, and O.E. Rimoldi, Stunning, hibernation, and assessment of myocardial viability. Circulation, 2008. 117(1): p. 103-114.
- ↑ Desai, M.Y., et al., Exercise echocardiography in asymptomatic HCM: exercise capacity, and not LV outflow tract gradient predicts long-term outcomes. JACC Cardiovasc Imaging, 2014. 7(1): p. 26-36.
- ↑ Aviles, R.J., et al., Utility of stress Doppler echocardiography in patients undergoing percutaneous mitral balloon valvotomy. J Am Soc Echocardiogr, 2001. 14(7): p. 676-81.
- ↑ Nishimura, R.A., et al., Low-output, low-gradient aortic stenosis in patients with depressed left ventricular systolic function: the clinical utility of the dobutamine challenge in the catheterization laboratory. Circulation, 2002. 106(7): p. 809-813.
- ↑ Paul, M., L. Smith, and M. Monaghan, Echocardiography. Medicine, 2010. 38(7): p. 371-375.
- ↑ 46.0 46.1 Ho, C.Y. and S.D. Solomon, A Clinician’s Guide to Tissue Doppler Imaging. Circulation, 2006. 113(10): p. e396-e398.
- ↑ Pratali, L., et al., Feasibility of real-time three-dimensional stress echocardiography: pharmacological and semi-supine exercise. Cardiovascular ultrasound, 2010. 8(1): p. 10-10.
- ↑ 48.0 48.1 Feigenbaum, H., Role of M-mode technique in today's echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography, 2010. 23(3): p. 240-257.
- ↑ 49.0 49.1 49.2 49.3 Lewis, J.F., et al., Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: clinical validation of two new methods using the apical window. Circulation, 1984. 70(3): p. 425-31.