Course:FNH200/2014w Team10 IceCream

Ice cream is a popular frozen dessert usually made from dairy products. It's popularity, especially among kids, may be attributed to its flavour (aroma and taste) and texture. There are currently over 100 ice cream flavours and probably many more to come! This page covers most elements that give ice cream its characteristic features. For example, think about what enables us to perfectly swirl ice cream on a cone and still be able to eat it cold on a hot summer day without spilling it on ourselves (well at least for the first few minutes).
Production

Milk fat, non-fat milk solids, sucrose, corn syrup solids, stabilizers, emulsifiers, and water are combined and blended to create an “ice cream mix”. The mix is then pasteurized to destroy pathogenic microorganisms and homogenized to decrease fat globule size in order to increase surface area of the cream mix. When the size of the fat globules is reduced and they are thoroughly blended, fat clumping is decreased giving a smoother cream texture. The ice cream mix is then aged for at least 4 hours inside a refrigerated compartment. Ageing allows fats to cool down from previous heat processes and crystallize resulting in a thicker consistency. Ageing also allows time for emulsifiers and stabilizers to increase their functionality. In the freezing and whipping process, the frozen mix is whipped in order to incorporated air which giving ice cream its light mouthfeel. Constant whipping of the mix also keeps the fat globule size small and allows to retain its smooth texture. Finally, the ice cream is hardened by cooling the mix to -30 to -40 degrees at which point the remaining water freezes.
Texture
Colloidal Dispersions in Ice Cream
Ice cream is a solid foam with gas as the dispersed phase and a solid as the continuous phase.The solid is usually a fat component such as cream or butter. It contributes not only to the richness of the flavour but also contributes to the ice cream’s smooth texture. The solid phase of the ice cream may also be comprised of non-fatty milk solids. They improve the body and texture as well as improve the ice cream's ability to hold air, the dispersed phase.
Ice cream may also be considered as an oil-in-water emulsion with the oil (fat) as the dispersed phase and water as the continuous phase.
Food Additives
Definition
In Canada, a food additive is, "Any chemical substance that is added to food during preparation or storage and either becomes a part of the food or affects its characteristics for the purpose of achieving a particular technical effect.” The use of a food additive must follow specific guidelines that ensure its safety and provide an advantage to the consumer. In Canada, it is strongly emphasized that that the food additive be advantageous to the consumer. Definition and examples of food additives in Canada
Lets take a look at the ingredient list of two varieties of Chapman's Ice Cream for examples of food additives.
Chapman’s original vanilla ice cream (4L)
Ingredients:Cream, Modified Milk Ingredients, Sugar, Glucose, Mono and Diglycerides, Locust Bean Gum, Cellulose Gum, Guar Gum, Carrageenan, Dextrose, Natural and Artificial Flavour
Chapmans no sugar added vanilla (lactose free) (1L)
Ingredients: Modified Milk Ingredients, Cream, Maltitol Syrup, Skim Milk Powder, Mono and Diglycerides, Guar Gum, Locust Bean Gum, Cellulose Gum, Carrageenan, Natural Flavour, Sucralose, Lactase. May contain peanuts and/or other nuts.
The above ingredient lists have the following food additives:
1. Stabilizers
Stabilizers are a group of polysaccharides, long chains of monosaccharide units, which contribute to the texture and viscosity of the unfrozen liquid in the ice cream. They do not allow water to move within the product giving ice cream a firmer and almost chewy texture. They also allow for smaller ice crystals because of their ability to hold water from migrating.
The absence of stabilizers would cause an increase in the size of ice crystals, due to water migration, and the product would turn coarse and icy at a much faster rate.
Examples

Carrageenan prevents the creaming of fat, separation of serum due to the incompatibility of different polysaccharide components with milk proteins, and stabilizes emulsions that may form.It is a polysaccharide extracted from red seaweed or irish moss. It strongly reacts with the proteins in milk to stabilize the mixture. It can form a wide variety of gel textures and thickens the mixture. It aids in providing a better texture and viscosity to ice cream.

Guar gum is a polysaccharide that comes from the guar plant. It is obtained in the form of a powder from the guar seeds and is added to foods during the production process. It is widely used and is known as one of the best thickening, stabilizing and emulsifying additives. In ice cream, it functions as a binder and stabilizer to reduce ice crystal formation and thus extend the shelf life of ice cream.
Cellulose gum is very abundant in nature as it is a polysaccharide found in all plants. It stabilizes and binds to water through hydrogen bonding which reduces the mobility of water. It prevents the formation of large ice crystals during temperature fluctuations.

Carob Bean Gum, also known as locust bean gum, is made from the seeds of a carob bean tree. It gives a creamy texture and reduces the growth of ice crystals in ice cream. It also functions as a water binder to reduce movement of water. It is usually used in combination with emulsifiers and is beneficial to use because of its neutral taste which does not affect the taste of the product.

2. Emulsifiers
Emulsifiers allow for the incorporation of air molecules within the fat globules in the ice cream resulting in a smooth texture and the "melt in your mouth" sensation characteristic to ice cream. They hold amphipathic properties, comprise of a polar and non-polar region, allowing it to interact with both water and fat and in the process diffusing the barrier between the two. Their main function in ice cream is to destabilize the fat, thus providing stable walls for air cells that are going to be formed. In the past, egg yolks were used as ice cream emulsifiers. Today, ice creams use mono and di-glycerides, products of the partial hydrolysis (break up) of fats from animals or vegetable oil, such as Polysorbate 80 as a food emulsifier.
Polysorbate 80 is synthesized from polyethoxylated sorbitan and oleic acid. It prevents the coating of fat droplets by milk proteins enabling the fat droplets to come together and capture air. As a result, a rigid structure is formed that holds the shape of ice cream even when it is brought to room temperature. The addition of polysorbate 80 allows the ice cream to melt much slower than it would otherwise.
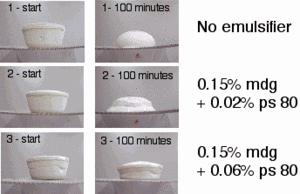
3. Sweeteners
Sweetening agents improve texture, palatability, flavours, and as a bonus, are a cheap way to contribute to the total amount of solids.
Examples
Lactose, naturally found in milk, is broken down by the lactase enzyme and acts by depressing the freezing point - that is, it helps water stay at its unfrozen state even at very low temperatures (-15 to -18 degrees) thus allowing ice cream to be scooped at these temperatures.
Maltitol is a sugar alcohol that is both naturally occurring and commercially processed. It has fewer calories and a lower glycemic index than sugar, but one of the highest glycemic indexes out of all the sugar alcohols. It is produced from the hydrogenation of maltose found in starch. This sugar alcohol is about 90% as sweet as sucrose.
Sucralose is a sugar substitute and is 600 times sweeter than sucrose. It is not metabolized by humans and hence does not add calories to the diet. It's molecular structure is similar to that of sucrose with the exception of three hydroxyl groups which are replaced by chlorine. This sweetener does not promote tooth decay and due to its sweetness only small amounts are required to be used.
Other examples not listed in the ingredient list above but may also be used as sweeteners in ice cream are listed below:
When used as a sweetener in ice cream, sucrose contributes to most of the sweetness. It is made up of two sugars: glucose and fructose. At concentrations above 10% sucrose, the molecule can be turned into “invert sugar” when treated with acid, water, and high heat. This increases the overall sweetness of the product.
Corn syrup can act as a complete or partial substitute for sucrose. It also creates a firmer, more chewy ice cream texture and improves the shelf life of the product. It is graded based on its dextrose equivalent (DE) which is a measurement of the syrups's reducing sugar content. When added to the ice cream mixture, corn syrup not only increases the sweetness but also the solute concentration which further increases the ability to retain unfrozen water at very low temperatures. A low DE, indicates a higher number of dextrins, products of partially hydrolyzed starches by acids or enzymes, which better stabilize the ice cream mix against a coarse texture. This attributes the smooth and creamy "mouthfeel" to a low-fat product which would otherwise need high amounts of to obtain the same sensation.
Acesulfame potassium is 200 times sweeter than sucrose, undigestible (therefore does not contribute to the caloric intake), and is stable to heat. This sweetener obtained through the transformation of acetoacetic acid and which is then combined with potassium. It is often used in combination with other sweeteners to enhance the sweet taste.
Sorbitol is a sugar alcohol that is commercially produced but is also naturally found in fruits. The source of commercially used sorbitol is the dextrose made in cornstarch, which is then hydrogenated to produce sorbitol. It is usually used as a bulk sweetener contributing about 1.5 – 3.0 kcal/g. Sorbitol is about 60% as sweet as sucrose.
Glycerine, also known as glycerol, occurs naturally in the body. It is a sugar alcohol and provides 4 calories per gram. It is only about 40% as sweet as sucrose and the main benefit of using it is that it has a very low glycemic index (3), compared to that of sugar (65). It is ideal for individual diagnosed with diabetes. It is used less for its sweetness and more for its property of keeping food moist.
Polydextrose is obtained from dextrose (glucose), it is more tart than sweet. It is mainly used to provide bulkiness and texture to food, without making the food sweeter. It is a low-caloric sweetener, yielding 1 kilocalorie per gram when digested. It also increases the non-dietary fibre content of the food.
Dextrose , naturally found in many food items, is a monosaccharide with the same molecular formula as glucose. However, glucose and dextrose differ in the way in which they attach to different molecules. Manufactures call glucose by the name of dextrose when it is produced from corn.
Depending upon the desired taste and nutritional characteristics, different varieties of sugars are used in ice cream production. In this example, 'Champan's Original Vanilla Ice Cream' uses '"sugar"' (most common form: sucrose), and glucose. While the 'Chapman's No Sugar Added (Lactose Free) Vanilla Ice Cream' uses maltitol syrup, over other sweeteners, because of its low caloric contribution as well as a low glycemic index especially since an additional sweetener such as sucralose, 600 times sweeter than sucrose, is also being used. It must also be acknowledged that, because of its "lactose free" characteristic, the ice cream may generally be sweeter than its original version.
4. Fats in Ice Cream
Fats act as lubricants to help the ice cream melt as desired when consumed. They contribute to the structure of the ice cream and play a significant role in the ageing, freezing, and aeration processes. For example, Chapman's uses cream (at least 10% milk fat). Some manufacturers, unlike Chapman's, use fat substitutes such as maltodextrin in ice cream production.
Maltodextrin is a carbohydrate based fat substitute, extracted from foods such as potatoes, corn, and tapioca. It is a mixture of glucose, maltose, oligosaccharides and polysaccharides. Maltodextrin is made through the partial hydrolysis of starch, by a bacterial enzyme, with further processing. It can replicate fat and provide bulk to foods. In ice cream, maltodextrin is used to maintain small ice crystal size and control the freezing point. It is ideal to use in ice cream production because of its solubility in cold water. Maltodextrin is fully digestible and yields 4 calories per gram.
All the above additives are permitted in Canada and are added with Good Manufacturing Practices (GMP). GMPs are put in place to ensure the proper regulation of food additives, that is, they must meet standards and be rightfully used.
Canadian Regulations
Standard Identity
According to the Food and Drug Regulations of Canada (B.08.062. [S].) ice cream is defined as and must follow the regulations below in order to be legally named ice cream.
It (a) shall be the frozen food obtained by freezing an ice cream mix, with or without the incorporation of air; (b) may contain cocoa or chocolate syrup, fruit, nuts or confections; (c) shall contain not less than 36 per cent solids,10 per cent milk fat, or, where cocoa or chocolate syrup, fruit, nuts, or confections have been added, eight per cent milk fat, and 180 grams of solids per litre of which amount not less than 50 grams shall be milk fat, or, where cocoa or chocolate syrup, fruit, nuts or confections have been added, 180 grams of solids per litre of which amount not less than 40 grams shall be milk fat; and (d) shall contain not more than 100,000 bacteria per gram and 10 coliform organisms per gram, as determined by official method MFO-2, Microbiological Examination of Ice Cream or Ice Milk, November 30, 1981. See Standard Identity of Ice Cream - Justice Laws Website
Labelling
The labelling regulations require the ice cream label to contain:
- Common name
- Net Quantity
- Name and address of person responsible for the product
- List of ingredients
- Nutrition facts table
- Durable life date
- Bilingual labelling
According to the CFIA, even though ice cream is a dairy product, the label is not required to show the percentage of milk fat present in the product.

Shelf Life
The shelf life of ice cream depends on how it is preserved and maintained. It can range from as long as two weeks to one year. It is important to to store ice cream under appropriate conditions in order to avoid the formation of large ice crystals that may damage the product; assuming that the ice cream has been produced under required conditions and comprises of ingredients that promote small ice crystal formation. The quicker the ice cream is frozen the better, and this can be done by using a barrel freezer. It is ideal to avoid major temperature changes during storage and distribution, since the processes of melting and freezing facilitate the formation of large ice crystals which decrease the quality of the ice cream. It is recommended, for the best texture, that ice cream stored at home should be eaten within a month.
Preservation Methods
Pasteurization
According to the regulations, the ice cream mix is pasteurized either at 68.3°C for 30 minutes or 79.4°C for 25 seconds. Ice cream pasteurization, unlike that of milk or other dairy products, requires higher temperatures and long time periods because of its increased viscosity which comes from higher amounts of fats, solids, sugar or sugar substitutes content, and the addition of egg yolks in certain ice creams or ice cream custard products.
Pasteurization sterilizes the ice cream mix thus rendering it free of pathogens and simultaneously inactivating the naturally occurring enzymes and microorganisms. This reduces the development of enzymatic byproducts and any fermentation that would otherwise naturally occur. Hence, flavour is preserved for longer and the shelf-life is extended.
Method
Ice Cream pasteurization generally occurs in a tank which is made up of a series of very thin stainless steel plates. Hot water (at 83°C) flows through one side of the plates and the cold ice cream mixture (at -37°C) goes through the other side of the plates allowing heat from the hot water to be transferred to the cold mix (bringing its temperature up to 82°C). Before the mixture has a chance to cool down, it goes through the process of homogenization.
Homogenization
Homogenization not only creates a uniform mix, but also reduces the size of the fat droplets, resulting in a stabilized emulsion. It results in a greater viscosity and in the production of a more uniform colour. It gives ice cream its creamy texture by breaking down large fat globules.
Method
The process of homogenization occurs in the homogenizer, which works like a piston pump by drawing up air and then forcing it out at a very high pressure. This pressure is used to force liquids through a very small tube like opening, creating very fine fat particles that prevent the separation of cream. In addition, this process effectively mixes all the ingredients, avoids disintegration of any soft materials and prevents the growth of harmful bacteria. Homogenization is necessary and important in the ice cream production, since it determines the reaction of the ice cream mix when it is frozen, hardened and distributed.
Packaging
Ice Cream is typically packaged in disposable waxed paper cartons or similar small sealed containers. This method prevents major problems such as heaviness when using metal cans, brittleness and exposure to light when using glass cans, or exposure to air leading to spoilage and contamination when using other types of containers. Nevertheless, no containers are a 100% free of contamination. Almost all of the commercialized bulk packaging is done in single-serve containers made of paperboard, plastic, or a variation of the two. Single-serve containers almost always have flattened sidewalls, bottoms, and rings that form the top and bottom. Ice cream tops are placed on directly after filling.
Dehydration
The process of freeze drying ice cream, is the same basic principle as that of other drying other food products. It is the process of removing water from the ice cream. For ice cream water is removed by lowering the air pressure to the point where the ice goes from a solid to a gas state. This process is enabled by placing the ice cream in a vacuum chamber and frozen until water crystallizes. Air pressure is lowered, creating a partial vacuum, forcing air out of the chamber, heat is applied and then a mechanism traps all the vaporized water. This enables freezing of the ice cream without letting the water melt, directly changing into a gas, preserving the molecular structure of the ice cream. This process repeats until freeze dried ice cream is created. See Freeze Dried Ice Cream
Freeze Dried Ice Cream is able to be called ice cream by Canadian Food Regulations due to its proper labelling of ingredients and in accordance with regulation 753 stating that a label and the use of “milk fat” is proper in compliance with the diary regulations. Regulations on ice cream are applied to Freeze Dried Ice Cream as Ice Cream is in its labelling. With ingredient complicate of regulation 753, Freeze Dried Ice Cream is allowed to be called Ice Cream. See Canadian Food Regulations
Use of Low Temperatures in the Production of Ice Cream
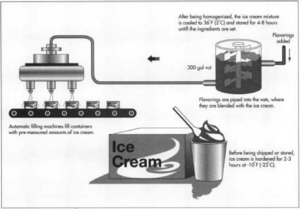
Once the mix has been homogenized, it is transferred to an equipment that is similar to a milk cooler. Next, it is poured (via pumps) into tank containers where it is kept for 4-8 hours, allowing the ingredients to set and harden. Ice cream mixtures can be left to set for a period of up to 24 hours. This time frame is known as “aging.”
Ice cream mixture can be frozen in single batch machines or continuous freezers. Both of these processes use the same type of machine, comprising of a cylindrical chamber with double walls that cool the refrigerant. There are rotating metal scraper, on the axis of the cylinder, that are used to remove any built up ice particles from the refrigerated walls.
Single Batch

The machine is filled halfway with the ice cream mixture after which it is turned on for the scrapers to continuously scrape for approximately 10 minutes until the mixture is chilled to (-5°C). At this point, when the volume is greatly increased as the scraper blades not only produce fine ice particles but also add air into the mixture, the cylinder opens and the batch mixture is quickly filled into containers were they are set to harden. Flavours, fruits, nuts and other ice cream additives are added right before the frozen ice cream is drained from the containers.
Continuous Freezing
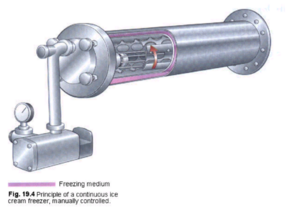
The ice cream mixture and a controlled volume of air is pressure pumped into horizontal jacketed tubes, each with their own rotating dasher and scraper. The mixture is slowly chilled to a temperature of (-6°C). while simultaneously the tubes are being vigorously agitated (process known as overrun) to create an informal freezing. This ensures the uniformity of the ice crystals and adds air to the mixture which attributes the characteristic lightness to ice cream. Once complete, the mixture will slowly be forced out and placed in containers that are taken to the hardening room to be frozen in batches at temperatures of about 0°F to -30°F which eventually form the ice cream.
Use Of Biotechnology in Ice Cream
Some Canadian Ice cream companies such as Chapman’s, use milk ingredients such as milk powder, whey powder and whey protein concentrate. This increases the milk protein concentration and results in a more smooth textured ice cream. Being the cheapest, whey protein concentrate is the most common form of whey protein, a byproduct of the cheese making process, which contains a high level of bioactive compounds. Whey protein is obtained by using simple drying methods, such as spray drying, to process whey, which is the leftover liquid mixture obtained through lactic acid production of the lactic acid bacterial culture after the milk coagulation process reaches a pH of 4.6 during cheese production.
Effects of Processing on Nutritive Values, Toxicants and Food borne Disease
With regards to the nutritive value of ice cream we must take into consideration the processing that occurs from the blending of the mix ingredients to the packaging, hardening and the final frozen dessert product. The pasteurization of the ice cream mix results in the loss of nutrient levels in the product. In order to achieve the 12D thermal process, the milk needs to be exposed to high temperatures for long times. Some loss of nutrients is inevitable in order to make the product safe for human consumption. In particular, the ice cream mix, and all other milk and dairy products, need to be pasteurized in order to eliminate the possibility of Escherichia coli, salmonella spp., and listeria monocytogenes, which are all bacterial toxins that are found in raw milk and dairy products. The 12D thermal process is a requirement that ensures these bacterial toxicants are removed from the milk before the continuation of the ice cream processing. Improper thermal processing of the product can lead to these bacterial toxicants remaining in the frozen dessert product despite being in its frozen state. E coli does not grow well below 8°C but cannot be killed by the low freezing temperatures, along with salmonella spp. Other toxicants to consider are those which are categorized as contaminates. Environmental toxicant contaminates include products of industrial activity, products of agricultural activity, products of food processing and naturally occurring environmental toxicants. Examples of these contaminants nclude traces of mercury, lead, pesticide residues and packaging residues. In order to ensure food safety and ensure your safety against foodborne disease, proper food handling and storage is recommended. Choosing foods that are pasteurized, keeping the frozen products blow an optimal -18°C and ensuring that this is a stable temperature to optimize the quality of the frozen dessert product. Due to the proactive efforts of the Canadian Food Inspection Agency (CFIA), Canadians have little to worry about as a result of the regulatory mechanisms in place that help insure that toxicant levels in our foods are kept below the no-effect level.
Exam Questions
1. Stabilizers in ice cream: a) Help to decrease water mobility, preventing large ice crystals from forming. b) Include carrageenan, guar gum, cellulose gum, and carob bean gum. c) Are all required to comply with good manufacturing practices (GMP). d) Also have emulsifying properties e) a. b. and c. only
2. What are the main components of ice cream that contribute to its characteristic texture? a) Homogenization: decreasing fat globule size and reducing fat clumping b) Emulsifiers: keeping air distribution and fat structure in the ice cream c) Stabilizers: preventing the migration of the unfrozen water in the ice cream d) All of the above
3. Why doesn’t ice cream completely freeze at low temperatures? a) the freezing methods were not done right b) the solutes decrease the freezing point by binding the free water so it only partially freezes c) the natural emulsifiers in the ice cream lower the freezing point of the mixture d) the packaging does not allow all the cold air to access the ice cream molecules
1. Correct answer: e. 2. Correct answer: d. 3. Correct answer: b.
Video Presentation
Click here for a video on how ice cream is made:
https://www.youtube.com/watch?v=C2_Gujz9lW8
This video highlights some differences, such as the absence of stabilizers, between industrial and homemade ice cream.
References
- Anthony, M. (2012, October 1). Understanding Polydextrose and How It Works. Retrieved March 31, 2015, from http://www.foodprocessing.com/articles/2012/understanding-polydextrose
- Carrageenan. (n.d.). Retrieved March 31, 2015, from http://www.agargel.com.br/carrageenan.html
- Chan, J. (Director) (2015, March 18). Lesson 11: Effects of Processing on Nutritive Values of Foods. Lecture conducted from, Vancouver.
- Chan, J. (Director) (2015, March 27). Lesson 12: Toxicants in Food and Foodborne Disease. Lecture conducted from, Vancouver.
- Dahle, C. (1931). The Effect of Pasteurizing and Homogenizing Temperatures on Certain Properties of Ice-Cream Mixes. Journal of Agricultural Research, 42(10), 675-688.
- Dairy Establishment Inspection Manual – Chapter 12 - Batch Pasteurization Tasks. (2014, February 14). Retrieved March 31, 2015, from http://www.inspection.gc.ca/food/dairy-products/manuals-inspection-procedures/dairy-establishment-inspection-manual/chapter-12/eng/1378105186699/1378105187387
- Everything You Need to Know About Acesulfame Potassium. (n.d.). Retrieved March 31, 2015, from http://www.nutritionexpress.com/article index/vitamins supplements a-z/showarticle.aspx?id=120
- Food Additives. (2013, October 24). Retrieved March 31, 2015, from http://www.hc-sc.gc.ca/fn-an/securit/addit/index-eng.php#a
- Food and Drug Regulations (C.R.C., c. 870). (2014, November 7). Retrieved March 31, 2015, from http://laws.justice.gc.ca/eng/regulations/C.R.C.,_c._870/page-111.html#h-78
- Food Grade Polysorbate 80. (n.d.). Retrieved March 31, 2015, from http://www.modernistpantry.com/polysorbate-80.html
- Food Gums. (n.d.). Retrieved April 1, 2015, from http://www.foodadditives.org/food_gums/common.html
- Galloway, E. (1968). Bulk Delivery, Storage and Dispensing Apparatus for Liquid Ice Cream Mixes and the Like. United States Patent Office, 1(1), 1-7.
- Glycerol. (n.d.). Retrieved March 31, 2015, from http://www.sugar-and-sweetener-guide.com/glycerol.html
- Goff, H. (n.d.). Ice Cream eBook. Retrieved March 31, 2015, from https://www.uoguelph.ca/foodscience/book-page/ice-cream-ebook
- Goff, H., & Hartel, R. (2013). Packaging, Hardening, and Shipping. In Ice cream (7th ed.). New York: Springer.
- Good Manufacturing Practices. (2015, February 27). Retrieved April 1, 2015, from http://www.hc-sc.gc.ca/dhp-mps/compli-conform/gmp-bpf/index-eng.php
- Guar Gum. (n.d.). Retrieved March 31, 2015, from http://www.guargum.biz/guargum_application.html
- How to Store Ice Cream Ice Cream. (n.d.). Retrieved April 1, 2015, from http://www.dairygoodness.ca/ice-cream/how-to-store-ice-cream
- Ice Cream Manufacture. (n.d.). Retrieved March 31, 2015, from https://www.uoguelph.ca/foodscience/book-page/ice-cream-manufacture
- Labelling Requirements for Dairy Products Percent (%) Milk Fat. (2014, December 22). Retrieved April 1, 2015, from http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/dairy-products/eng/1393082289862/1393082368941?chap=9
- Locust Bean Gum. (n.d.). Retrieved April 1, 2015, from http://ingredientssolutions.com/locust-bean-gum/
- Locust bean gum. (n.d.). Retrieved April 1, 2015, from http://www.danisco.com/product-range/locust-bean-gum/
- Ludvigsen, H. (2011, October). Manufacturing high quality ice cream with high overrun. Retrieved March 31, 2015, from http://www.palsgaard.com/media/172509/ice%20cream%20with%20high%20overrun.pdf
- Maltitol. (n.d.). Retrieved April 1, 2015, from http://www.sugar-and-sweetener-guide.com/maltitol.html
- Maltitol. (n.d.). Retrieved April 1, 2015, from http://www.caloriecontrol.org/sweeteners-and-lite/polyols/maltitol
- Maltodextrin. (n.d.). Retrieved March 31, 2015, from http://www.c-c-l.com/ingredients,maltodextrin.htm
- Maltodextrins and corn syrup solids. (n.d.). Retrieved March 31, 2015, from http://www.grainprocessing.com/food/frozen-desserts.html
- Maynard, M. (Ed.). (2012, September 4). Gelling agents, thickeners & stabilisers. Retrieved April 1, 2015, from http://www.faia.org.uk/gelling-agents-thickeners-stabilisers/
- Sucralose Benefits. (n.d.). Retrieved March 31, 2015, from http://sucralose.org/benefits/
- Sugar Alcohols. (n.d.). Retrieved March 31, 2015, from http://www.sugar.org/other-sweeteners/sugar-alcohols/
- Sugar Alcohols (Polyols) & Polydextrose used as Sweeteners in foods. (2005, February 16). Retrieved March 31, 2015, from http://www.hc-sc.gc.ca/fn-an/securit/addit/sweeten-edulcor/polyols_polydextose_factsheet-polyols_polydextose_fiche-eng.php
- Tharp, B., & Young, S. (1997, October 1). On Ice Cream. Retrieved March 31, 2015, from http://www.onicecream.com/qa_pasthom.html
- What Is the Difference Between Dextrose, Fructose, and Glucose? (n.d.). Retrieved April 1, 2015, from http://www.wisegeek.org/what-is-the-difference-between-dextrose-fructose-and-glucose.htm
- What You Need to Know About the Four Types of Sugar in Food. (2010, September 21). Retrieved April 1, 2015, from http://www.goodhousekeeping.com/food-recipes/healthy/a18910/types-of-sugar-0921/
- www.chapmans.ca
- http://www.dairyinfo.gc.ca/index_e.php?s1=dr-rl&s2=canada&s3=ndc-cnpl&s4=05-2005
- https://www.youtube.com/watch?v=k1pel10ufmk
- http://www.hc-sc.gc.ca/fn-an/securit/addit/index-eng.php#a1
- http://laws.justice.gc.ca/eng/regulations/C.R.C.,_c._870/page-111.html#h-78
- Ice Cream Fats. (n.d) Retrieved March 20, 2015, from http://www.aak.com/Global/Brochures/Ice%20Cream%20AAK.pdf
- From Cow to Cone. How Ice Cream is made. (n.d.). Retrieved March 20, 2015, from http://www.idfa.org/news-views/media-kits/ice-cream/from-the-cow-to-the-cone
- Goff, H. (2014, April 21). Ice Cream Mix Ingredients. Retrieved March 17, 2015, from https://www.uoguelph.ca/foodscience/book-page/ice-cream-mix-ingredients