Course:FNH200/2011w Team20 Vanilla
Introduction
Vanilla can be extracted from the vanilla bean through many processes. Some of which require alcohol and some do not. Vanilla is not only found in baked goods but also in ice cream, beverages, perfumes and pharmaceuticals. Extracting vanilla targets 4 main molecules, the most common molecule is called vanillin, is extracted and used in the production of vanilla extract and flavouring. Vanillin can also be used as a food preservative from spoilage-causing bacteria, yeast and mould. [1] Vanillin can be extracted in many ways but which extraction process reaps the highest quality of vanilla extract? The four main extraction methods will be discuss below. Three of which use alcohol in the extraction process and one of which is non-alcoholic. We have picked one non alcoholic because it is the only method known to date that does not use ethanol or any other solvent that contains an alcohol functional group.
History of the Vanilla Bean

Vanilla has been one of the world's most favourite flavouring material since the 16th century. Bernal Diaz, a spanish officer under the supervision of Hernando Cortez, discovered vanilla when he observed the Aztec emperor drinking a beverage that contained vanilla. The Aztecs were extremely guarded about their secret of vanilla but the Spaniards found a way to import it to Spain and managed to establish factories in the late 16th century. In the 16th century, vanilla was only consumed by the wealthy and noble in forms of beverages and chocolate. Vanilla was very hard to cultivate in other areas of the world until the discovery of artificial pollination by Charles Morren.[2] The Vanilla orchid requires 3-4 years to set and only flowers once a year. The vanilla bean is then left for 8-10 months to develop before harvesting.[3] When the beans are harvested, they are treated with hot water or heat and are then placed in the sun every day for weeks-to-months until they have shrunk to 20% of their original size. [4] Vanilla expanded its uses and was commonly used for its healing qualities for fevers and spasms. Today, vanilla is a very popular scent in fragrances. [2] and vanilla beans are commonly found in tropical climbing orchids. The largest producers of vanilla are Mexico, Madagascar, Indonesia and Tahiti. [1]
Vanilla Extraction Processes
Alcoholic Extraction Methods
The best homemade extraction for vanilla is as follows:
- Spilt the vanilla bean
- Add the beans and seeds to a jar and add alcohol (Vodka)
- Shake and store in a dark dry location
- Shake and check every so often for the next 4 - 6 weeks
- Optional: strain the beans or leave them in the jar
Commercial Extraction
Natural vanilla extract, a dilute ethanolic extract, is commercially sold containing approximately 1.0g/l vanillin. Commercial vanilla extract, a dilute ethanolic extract, has two forms of extraction. The first is the percolation method resulting in a four-fold strength vanilla extract, a fold being the concentration of the extract. The extractable material of a single fold of vanilla extract contains 100g of vanilla pods per liter. It takes 48-72 hours, and consists of a mixture of water and ethanol which contains 35-50% alcohol. The second method is the oleoresin method resulting in a vanilla extract that is 10-fold in strength. However, the time for this extraction is much greater, taking about 89 days. The process involves pulverizing whole vanilla pods and under a vacuum at 458°C, circulating ethanol over the pods. Evaporation eliminates the excess alcohol. The color of vanilla extract varies depending on the quality of the pods, the length of the extraction, the alcoholic strength, and the added glycerin used to slow down the evaporation and give the extract flavour. [5]
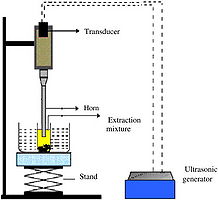
Ultrasound Assisted Extraction
Ultrasound aids in mass transfer from the bean to the solvent (solid to liquid form). Ethanol is the best for extraction of vanilla because "like dissolves like". Meaning polar molecules dissolves polar molecules since ethanol and vanilla beans are both polar. When it comes to extraction quality trumps quantity. When placing 1g of good quality vanilla beans in ethanol more vanillin yields are gained than when placing 3g of poor quality vanilla bean in ethanol. Ultrasound extraction can be accelerated by increasing the temperature in which the reaction occurs. An increase in temperature leads to an increase in rate of recirculation of the solvent through the vanilla beans therefore increasing the extent of extraction. With ultrasound extraction theres a maximum threshold of extraction that is usually reach after one hour of the extraction process. Even through that is a short period of time, it still extracts more vanillin in one hour vs. four hours of the conventional soxhlet extraction. How it works is by increase the rate of diffusion and decreasing the permeability of the cell membrane of the beans. Another helpful aid in the extraction process is to increase the ethanol to water ratio of the solvent. The more ethanol the solvent contains the more efficient the extraction process will be. [1]
Conventional Extraction
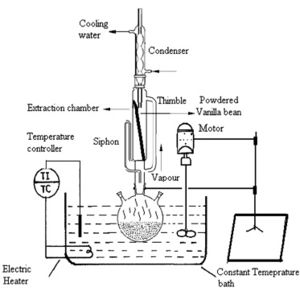
There are many forms of conventional methods for the extraction of vanilla. Some of these include soxhlet, heat treatment, maceraction and homogenization.[5] These methods allow for the determination of the most convenient temperatures and compounds used in the larger scale vanilla extraction processes. The Soxhlet extraction process is one of the conventional methods of extracting vanilla from the vanilla pods. The temperature of the process is generally kept around 95°C, while the ratio of solvent to solute is 200 ml to 66.67 ml/g. The process begins with powdered cured vanilla beans that are placed in a thimble holder. Due to the arrangement of the apparatus, the reservoir generates vapors of the solvent that becomes condensed in the condenser after it passes through the thimble. The extraction occurs at the thimble when the condensed fresh solvent comes in contact with the cured vanilla beans. The liquid moves through a siphon carrying extracted solutes into the bulk liquid and back into the reservoir once it reaches the overflow level in the thimble. Distillation is used to separate the solute from the solvent, which occurs in the solvent flask. After the completion of the distillation process, solvent vapours pass back through the condenser for another cycle of extraction and the solute remains in the flask. [1] Through the process, the most effective solvent, which achieves the maximum yield of vanilla extract, is ethanol. There are however, drawbacks to conventional methods such as large extraction times, low extraction yields and higher solvent consumption.[5]
Non-Alcoholic Extraction Methods
Glycerol-Based Extraction

Glycerol (propane-1,2,3-triol) also known as glycerin is a polypol compound that is colorless, odorless and viscous. The natural texture of vanilla tends to be darker in colour. The primary uses of glycerol range from food and beverages to toiletries. When extracting vanilla from vanilla beans non-alcoholically, glycerin is used to as a substitute for alcohol. Glycerin can play many roles in food products. It can be used as a sweetner, preservative or a thickening agent. Since glycerin is non-toxic at many levels of ingestion it can be used in baked goods and drugs. Another added benefit of glycerin is that although it is 60% as sweet as sucrose, it does not promote formation of oral cavities or tooth decay. [6] The non-alcoholic process involves the longitudinal opening of the vanilla bean and scrapping of the seeds. Then, both the bean and seeds are placed in a solution of glycerin and warm water, and stored in a dark area. The process takes up approximately one month and requires constant shaking of the content before it is ready for use. It is optional to strain the beans after one month to produce a homogenous product.
Summary of All Extraction Processes
| Extraction Method | Temperature | Time | Content |
|---|---|---|---|
| Alcoholic Extraction | 20-25°C | 3-4 months | Vanilla beans and vodka |
| Commercial Extraction | ~458°C | Ranges from 2 to 89 days | Water and ethanol (30-35% alcohol) |
| Conventional Extraction | 95°C | 4 hours | Vanilla beans (66.67 ml/g) and ethanol (200 ml) |
| Ultrasound Assisted Extraction | N/A | 1 hour | Vanilla beans (1g) and ethanol |
| Non-alcoholic Extraction | 20-25°C | 1 month | Vanilla beans and seeds, glycerol and water |
Alcoholic vs. Non-alcoholic Vanilla Extract
The uses of alcoholic vanilla extracts are commonly found in food products, fragrance, hygienic products and toiletries. This may be due to the low boiling point of ethanol, 78°C, which is also volatile. This makes it suitable for such hygiene products. For example, perfumes that use glycerol-based vanilla extract would be greasy to the touch and would lose their aromatic properties. The three discussed alcoholic extractions either require the same amount of time as non-alcoholic or less time. Ultrasound Assisted Extraction is the fastest of the alcoholic methods and commercial is the slowest because it can take up to 89 days for the extraction process to be complete. In contrast, non-alcoholic vanilla extract can be found in pharmaceuticals, drugs and food products. It is important to note that glycerol is a fat and carbohydrate based molecule, which gives it a high boiling point of 171°C. This high boiling point gives it an advantage of being used in products that require heating to high temperatures for processing reasons such as pharmaceuticals and drugs. Two disadvantages of non-alcoholic are a slight after taste but generally similar flavor and longer extraction time.
| Types | Temperature | Time | Uses | Disadvantages |
|---|---|---|---|---|
| Non-Alcoholic | B.P. of 171°C | Long extraction time | Pharmaceuticals and food | Odd aftertaste |
| Alcholic | B.P. of 78°C | Short extraction time | Food, fragrances and hygenic products | Allergy and intoxication |
Intoxication and Allergy

Vanilla extract containing ethanol can have many serious effects if ingested in high amounts. Some effects are liver damage, hypotension, low body temperature (hypothermia), increased heart rate, nervous system depression, gastrointestinal distress and dilated pupils.
[7] Vanillin, which is found in vanilla extract, is known to cause a range of allergic reactions for about 2.6% of people. The most common reaction is dermatitis which is an eczema type rash.[8]. Alcoholic vanilla extracts can cause problems for some people that have a sensitivity or allergy to alcohol. These people can have reactions that include flushing syndrome, anaphylactoid reactions, triggers asthma and many more [9]. However, for people who cannot consume alcohol, there is an alternative for vanilla extract where they substitute the alcohol for glycerol. Glycerol has several uses and is proven to be nontoxic, non-irritating and odorless.[6]
Conclusion
Vanilla extract has been used over several centuries for things such as food flavoring, scents in fragrances and for its healing properties. Vanilla was discovered in Mexico and continues to be cultivated there as well as other places that have a warm climate. Vanilla can be extracted using several different methods but we focused on alcoholic extraction including commercial extraction, conventional extraction and ultrasound assisted extraction as well as another form of non-alcoholic extraction. Not much research was found as to which intensive purposes each extraction method was used but as a group we deduced these assumptions based on scientific evidence as to why each method is used. The Conventional Soxhlet extraction is most likely used in laboratories for testing and scientific purposes. We made this assumption because it produces vanilla extract in small quantities on a small scale. This method would be ideal for pharmacological testing, drugs and by food scientists who are inventing new food compounds. Commercial extraction is an internationally used method of vanilla extraction because it can produce vanilla extract on a wide scale in an efficient manner. For this reason we believe commercial extraction is the most commonly used method used to produce vanilla extract that we find at our local supermarkets. Ultrasound Assisted Extraction is an even more efficient way of extracting vanillin from the vanilla pods that would take a fraction of the time of commercial extraction. This method uses ultrasound waves to speed up the rate of reaction. Due to high temperatures some denaturation of the chemical compounds of the vanilla bean and the solvent may occur. Loss of volatile acids and oils which correspond to a loss of flavour and aroma. This helps create a more concentrated vanilla extract but that does not necessarily mean the highest quality of the extract. With the glycerin based extraction we believe the best vanilla flavour would be found in this mixture because glycerin is a fatty acid and will adhere to flavouring particles much stronger. Moreover, glycerin has a naturally sweet taste which will add to the vanilla flavour making it sweeter. This may cause the slight after taste and may slightly change the flavouring effect of the extract. Our group discussed and decided that we preferred the non-alcoholic extraction method the best because the alcohol in regular vanilla extract is replaced with a glycerol substitute. The non-alcoholic vanilla can therefore withstand higher temperatures when used in cooking as well as contribute to the manufacturing of pharmaceutical medications. Vanilla extract that contains alcohol can also cause problems for some people if they have an allergy to alcohol or consume too much vanilla extract. In these situations, the non alcoholic vanilla extract is a better choice because glycerol is know to be a non-irritating and non toxic.
| Method | Some Uses | Example Products |
|---|---|---|
| Commercial | Food products | Chocolate chip cookies |
| Conventional | Pharmaceuticals | Lip glosses and lip balm |
| UAE | Drugs | Chewable multivitamins |
| Non-Alcoholic | Food products | Ice cream |
References
- ↑ 1.0 1.1 1.2 1.3 Jadhav, D., Rekha, B.N., Gogate, P.R., & Rathod,V.K.(2009).Extraction of vanillin from vanilla pods: a comparision study of conventional soxlet and ultrasound assisted extraction. Journal of food Engineering, 93,421-426. doi:10.1016/j.jfoodeng.2009.02.007
- ↑ 2.0 2.1 Correl, D.S. (1953). Vanilla- its botany, history, cultivation and economic import. Economic Botany, 7, 291-358. Retrieved from http://www.jstor.org/stable/4287786?seq=1&Search=yes&searchText=alcohol&searchText=vanilla&searchText=extract&list=hide&searchUri=%2Faction%2FdoBasicSearch%3FQuery%3Dvanilla%2Bextract%2Balcohol%26acc%3Don%26wc%3Don&prevSearch=&item=3&ttl=170&returnArticleService=showFullText&resultsServiceName=null
- ↑ Havkin-Frenkel, D., French, J.C., Graft, N.M., Joel, D.M., Pak, F.E., & Frenkel, C. (2004). Interrelation of curing and botany in vanilla (Vanilla planifolia) Bean. Acta Horticulturae (ISHS), 629, 93-102. Retrieved from http://wwwlib.teiep.gr/images/stories/acta/Acta%20629/629_12.pdf
- ↑ ServoLux B.V. (2007). Vanilla Extracts. Retrieved from http://vanilla.servolux.nl/vanilla_extracts.html
- ↑ 5.0 5.1 5.2 Sinha, A.K., Sharma, U.K., Sharma, N. (2008) A comprehensive review on vanilla flavour: extraction, isolation and quantification of vanillin and others consitituents. International Journal of Food Sciences and Nutrition, 59, 299-326. Doi:10.1080/09687630701539350
- ↑ 6.0 6.1 http://www.biodiesel.org/resources/reportsdatabase/reports/gen/19960901_GEN-051.pdf
- ↑ Mazor, S.S., DesLauriers, C.A., & Mycyk, M.B. (2005). Adolecent ethanol intoxication from vanilla extract ingestion: a case report. The international journal of family practice, 4, 1. Retrieved from http://www.ispub.com/journal/the-internet-journal-of-family-practice/volume-4-number-1/adolescent-ethanol-intoxication-from-vanilla-extract-ingestion-a-case-report.html
- ↑ Niinimaki, A. (1984). Delayed-type allergy to species. Contact Dermatitis, 11, 34-40. dio:10.1111/j.1600-0536.1984.tb00168.x/pdf
- ↑ Gonzalez-Quintela, A., Vidal, C., & Gude, F. (2004). Alcohol, IgF and allergy. Addiction Biology, 9, 195-204. dio: 10.1111/j.1369-1600.2004.tb00533.x